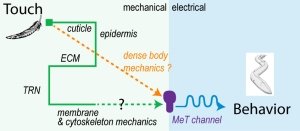
- Sanfeliu-Cerdan and Krieg. The mechanobiology of biomolecular condensates. Biophysics Reviews. 2025 pdf
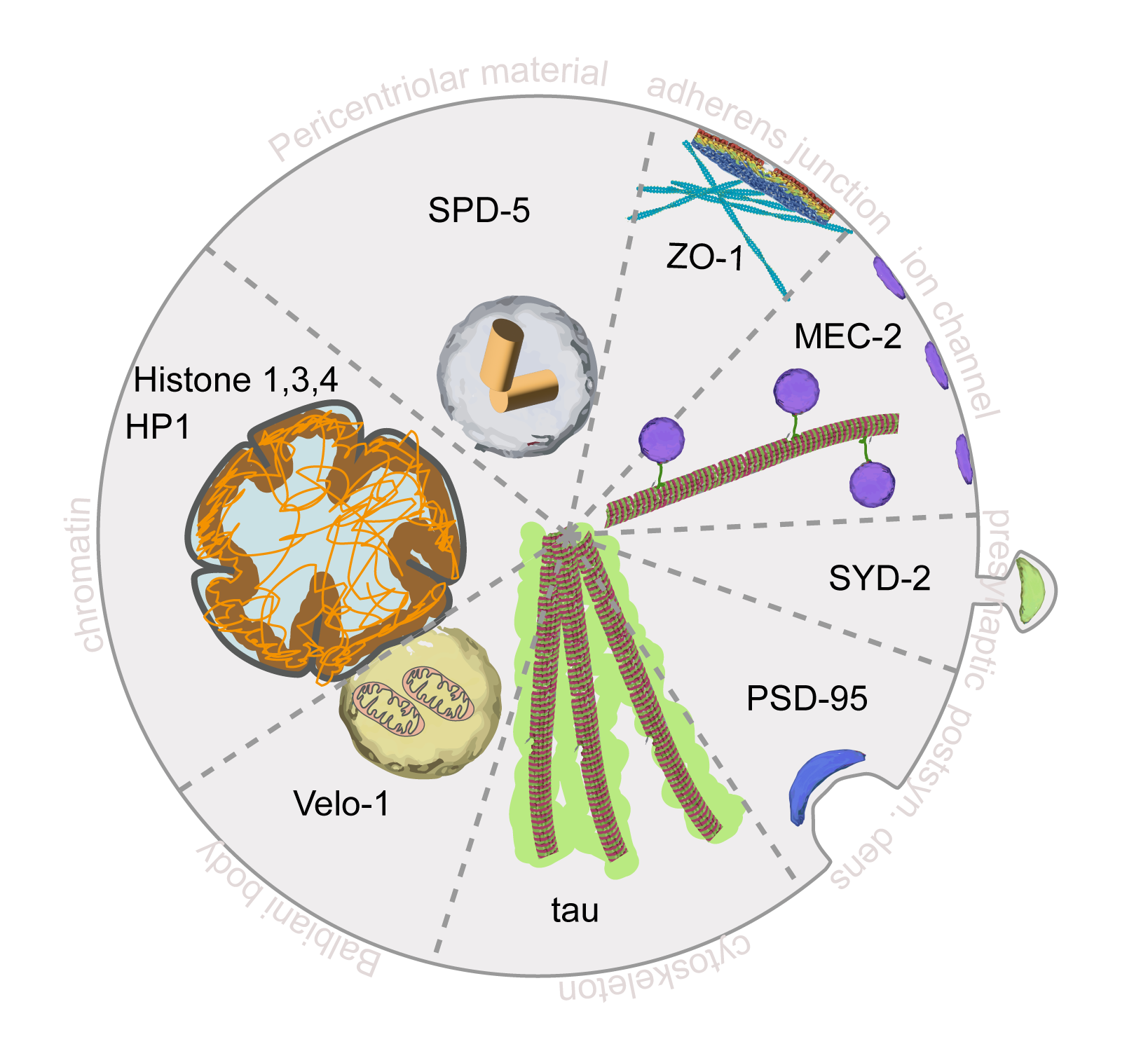
- Catala-Castro; Bonilla-Quintana. Periodic Obstacles Regulate Membrane Tension Propagation to Enable Localized Mechanotransduction. BioRxiv, 2025
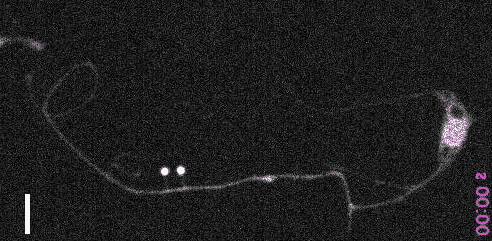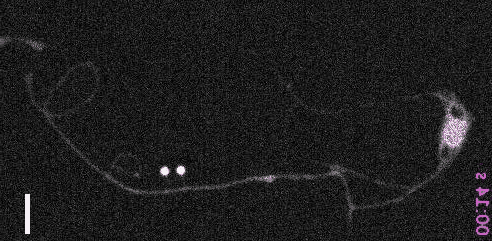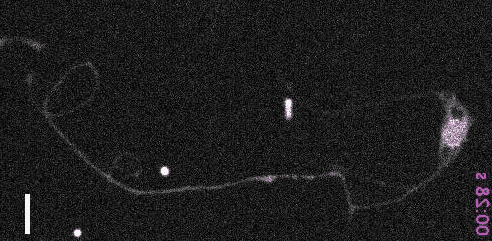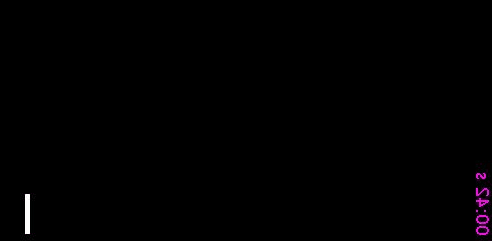
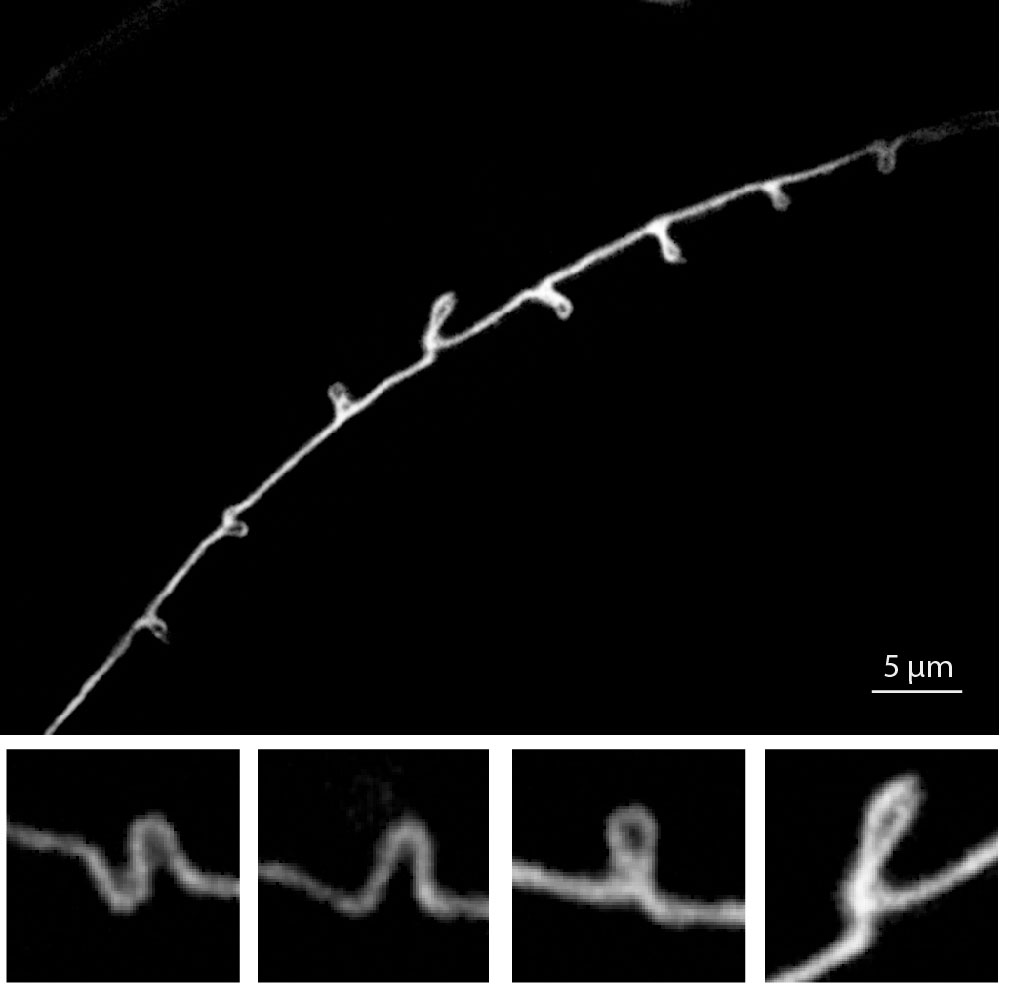
- Inberg, et al. Sensory experience controls dendritic structure and behavior by distinct pathways involving degenerins. eLife, 2025

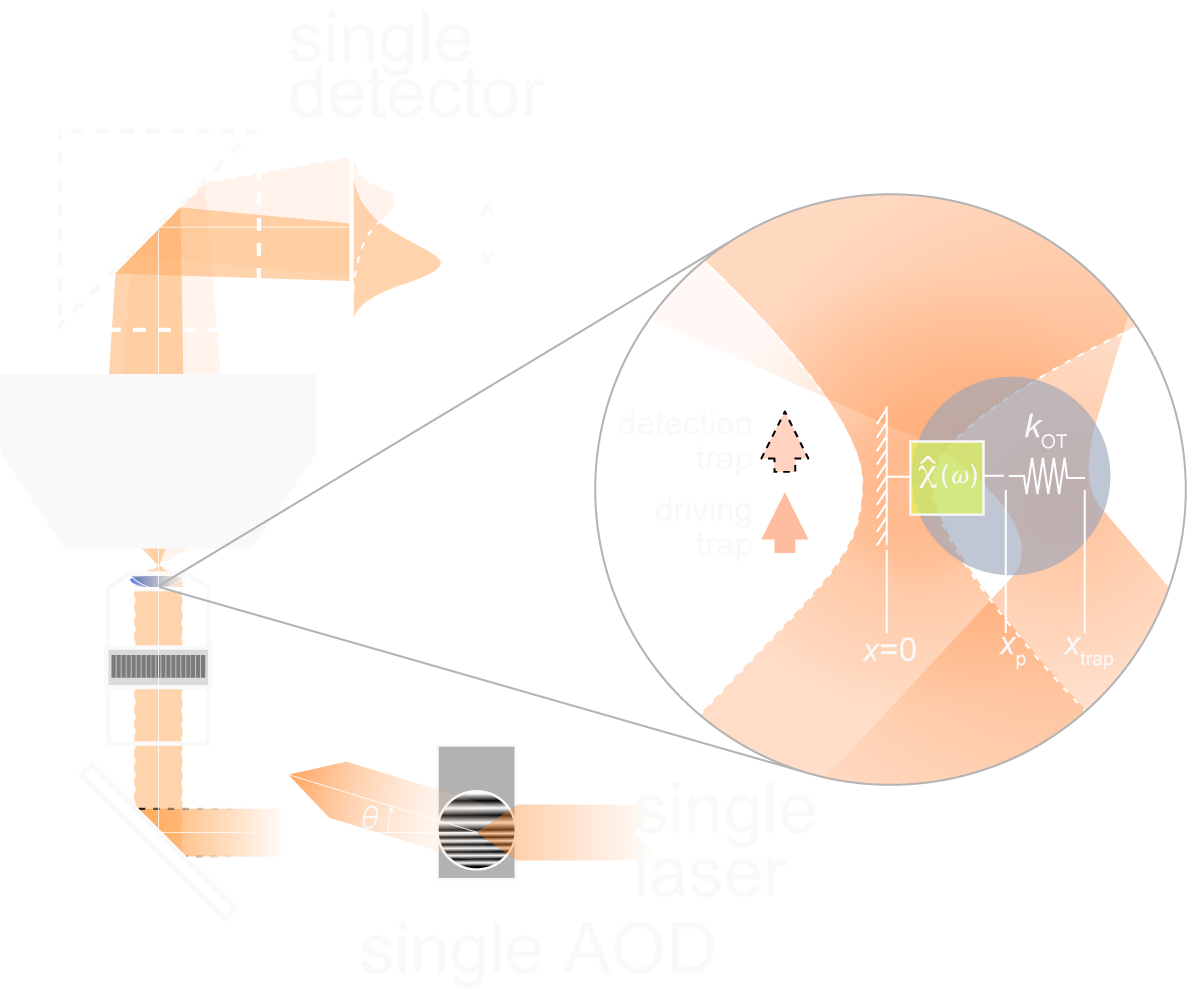
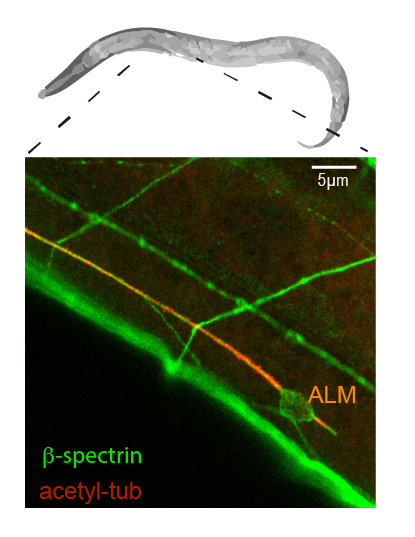
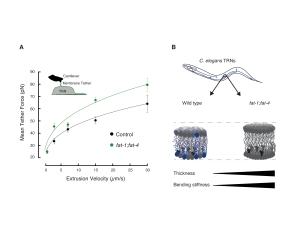
Malaiwong et al. Mechanical load conditions the spectrin network to run on proteolysis and early on set neurodegeneration. BioRxiv. 2024- Catala-Castro; Ortiz-Vasquez, Fernandez-Martinez et al. Measureing age-dependent viscoelastic properties of organelles, cells and organisms via Time-Shared-Optical Tweezer Microrheology (TimSom). Nature Nanotechnology, 2025, pdf
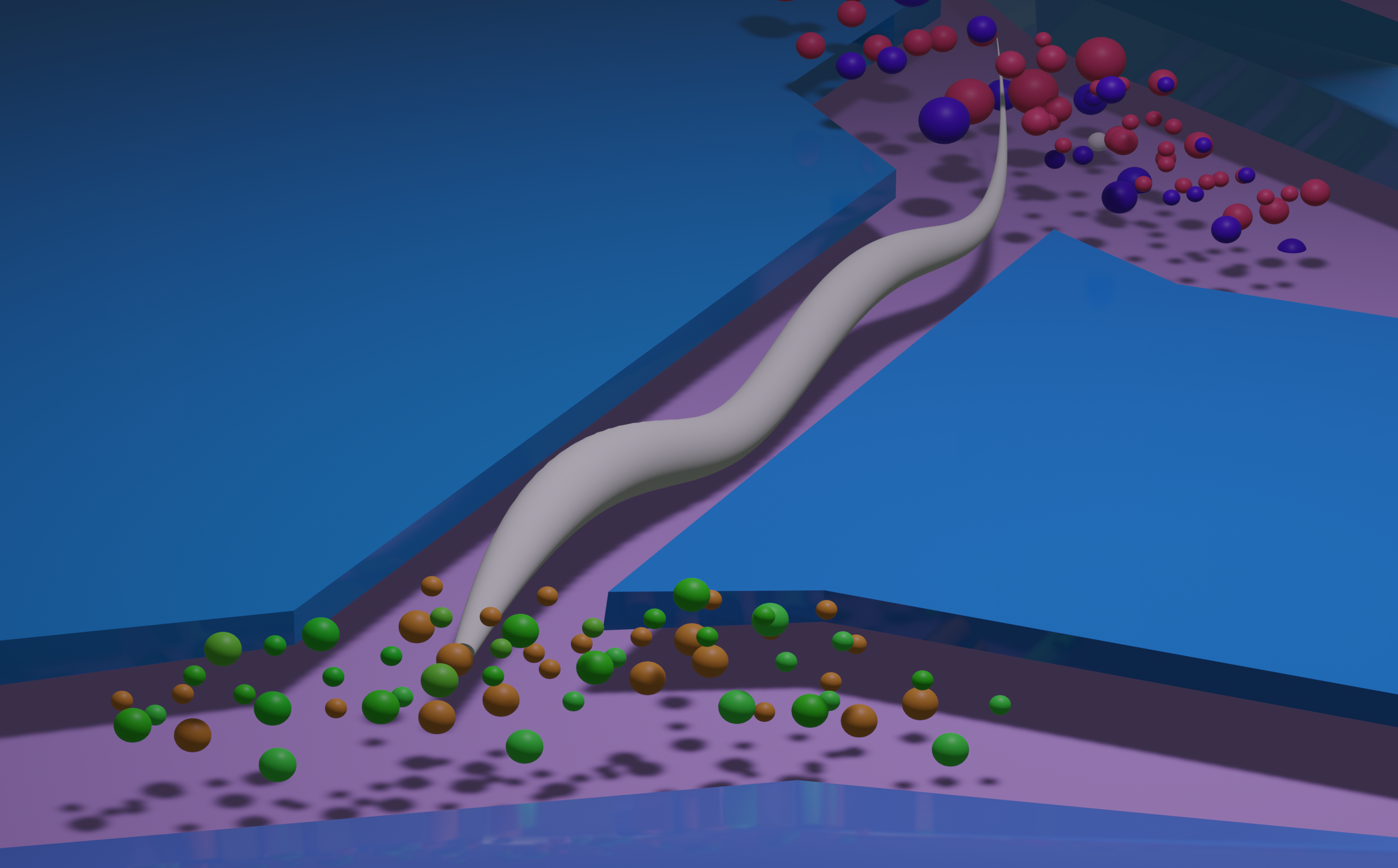
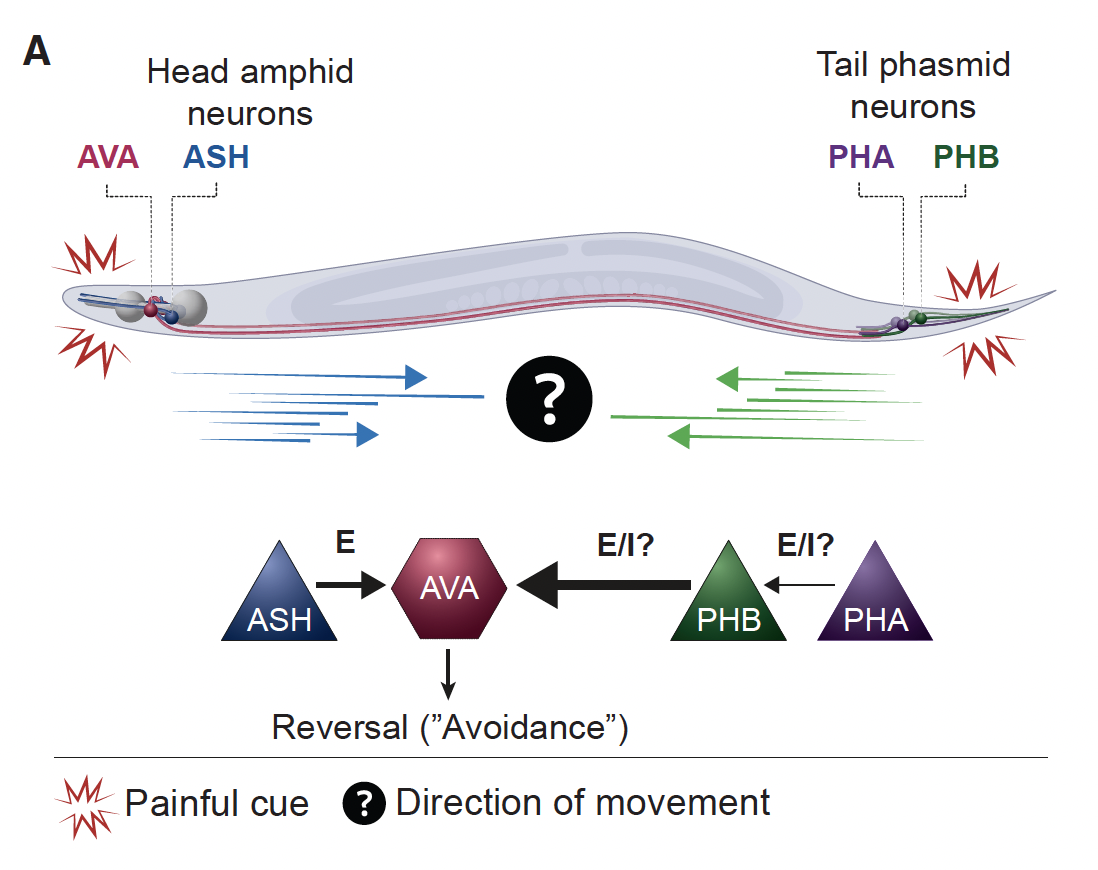
- Karimi et al. Automated Dual Olfactory Device for Studying Head/Tail Chemosensation in Caenorhabditis elegans. APL BioEngineering. 2024 pdf
 see also the APL SciLight feature here: https://doi.org/10.1063/10.0025863
see also the APL SciLight feature here: https://doi.org/10.1063/10.0025863 - Porta-de-la-Riva et al. Bioluminescence as a functional tool for visualizing and controlling neuronal activity in vivo. Neurophotonics. 2024. pdf
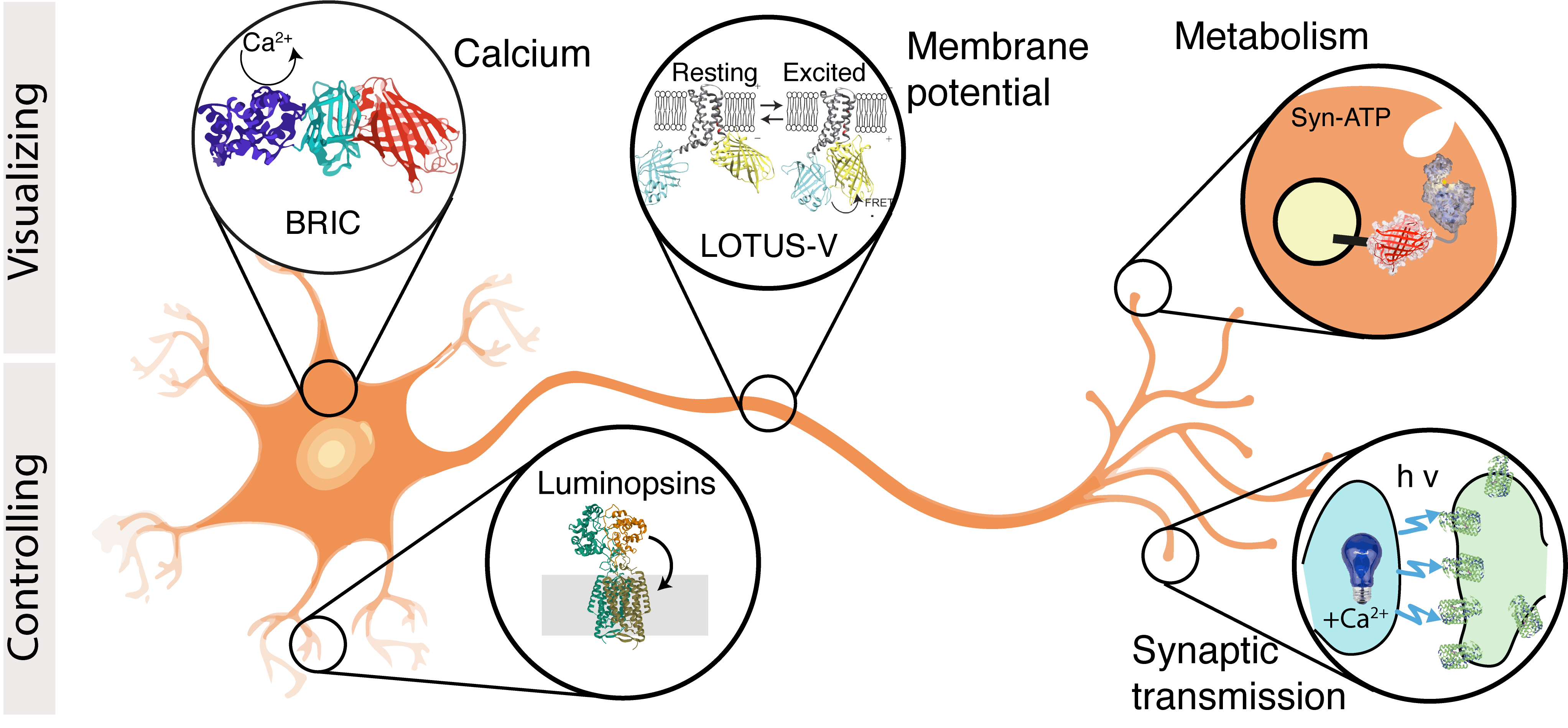
- I Rabinowitch, DA Colón-Ramos, M Krieg, Understanding neural circuit function through synaptic engineering. Nature Reviews Neuroscience, 2024 pdf cover
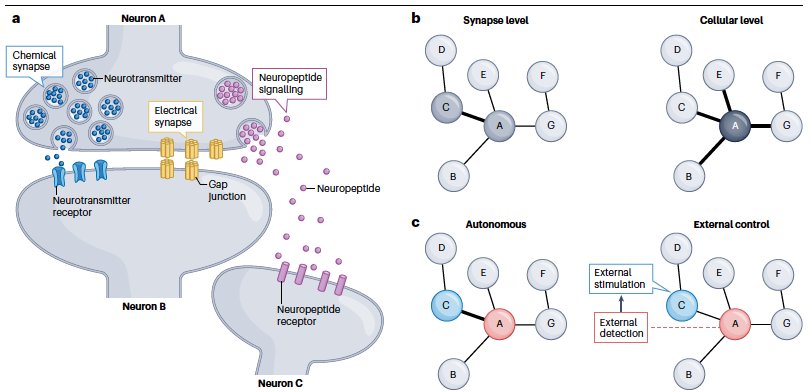
- Gat et al. Integration of spatially opposing cues by a single interneuron guides decision making in C. elegans. Cell Reports, 2023 pdf
- Porta-de-la-Riva et al. Neural engineering with photons as synaptic transmitters. Nature Methods. 2023 si material link
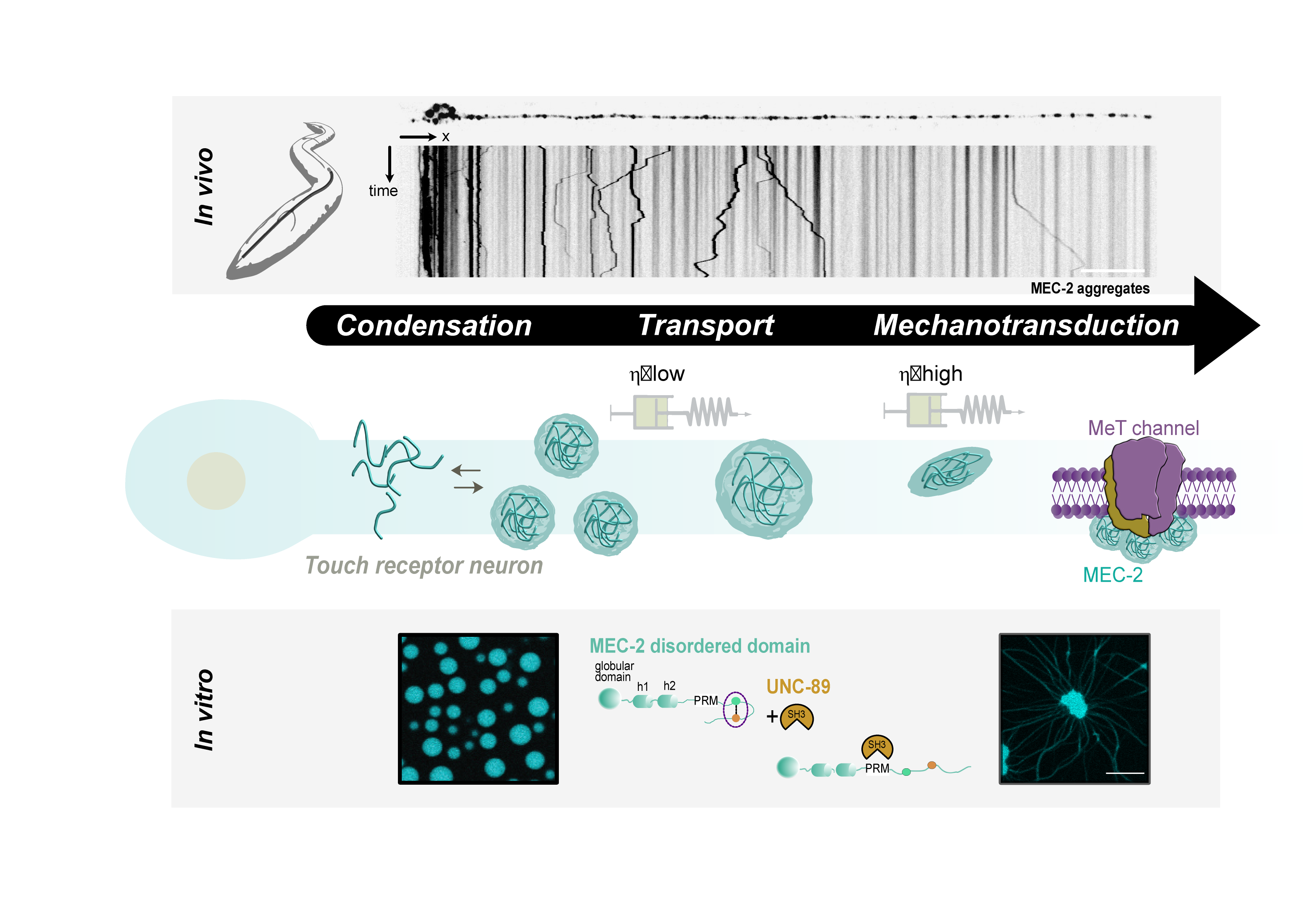
- Sanfeliu et al. A MEC-2/Stomatin condensate liquid-to-solid phase transition controls neuronal mechanotransduction during touch sensing. Nature Cell Biology. 2023. pdf
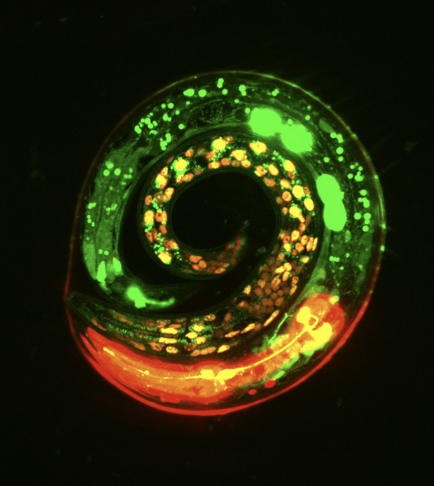
- Malaiwong et al. FLInt: Single Shot Safe Harbor Transgene Integration via Fluorescent Landmark Interference. G3: Genes|Genomes|Genetics. 2023. pdf
- Sanfeliu et al. Visualizing neurons under tension in vivo with optogenetic molecular force sensors. Methods in molecular biology, 2023 pdf
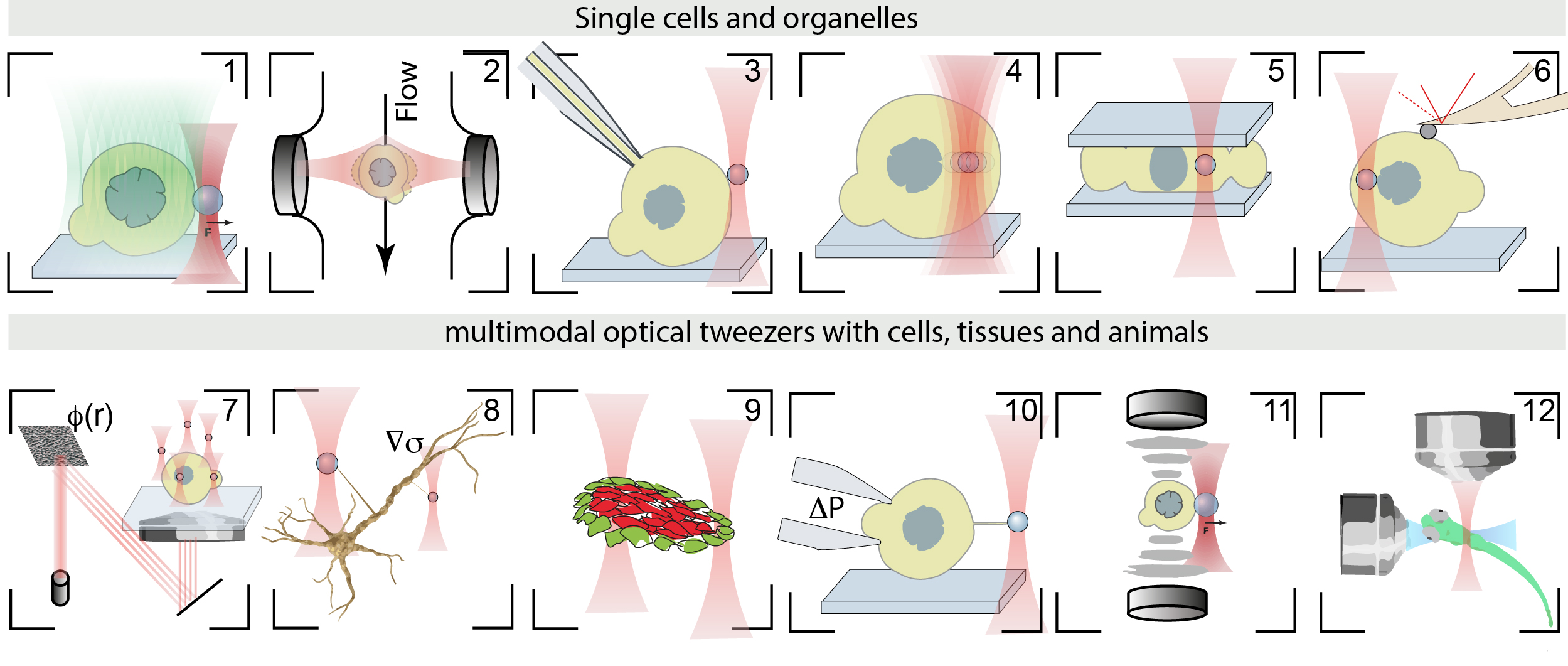
- Catala-Castro, et al. Exploring cell and tissue mechanics with optical tweezers, Journal of Cell Science, 2022 pdf
- Curiel, et al. Volumetric bioluminescence imaging of cellular dynamics with deep learning based light-field reconstruction, Communications Biology, 2022 pdf

- Falconieri, et al. Axonal plasticity in response to active forces generated through magnetic nano-pulling. Cell Reports, 2022 pdf
- Setty et al. Sexually dimorphic architecture and function of a mechanosensory circuit in C. elegans. Nature Communications, 2022 pdf
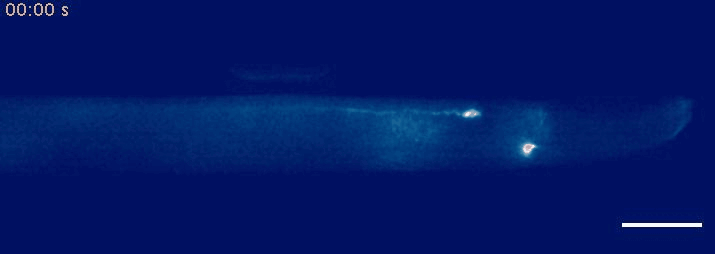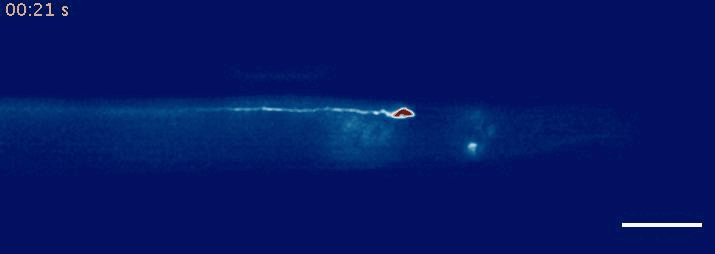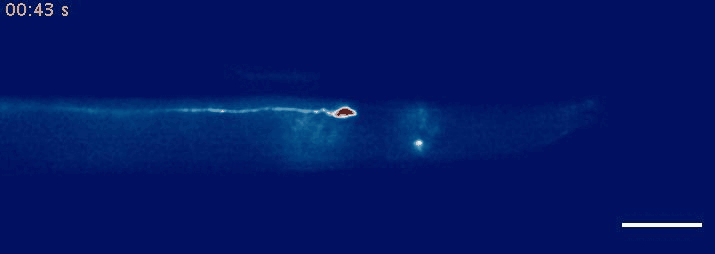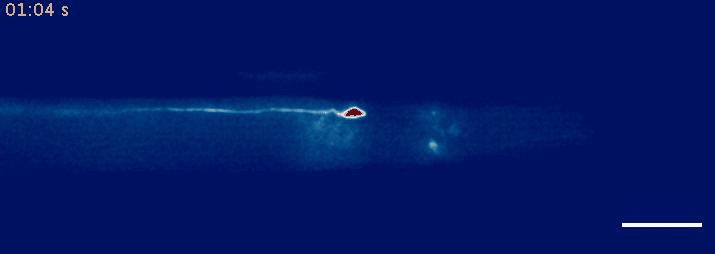
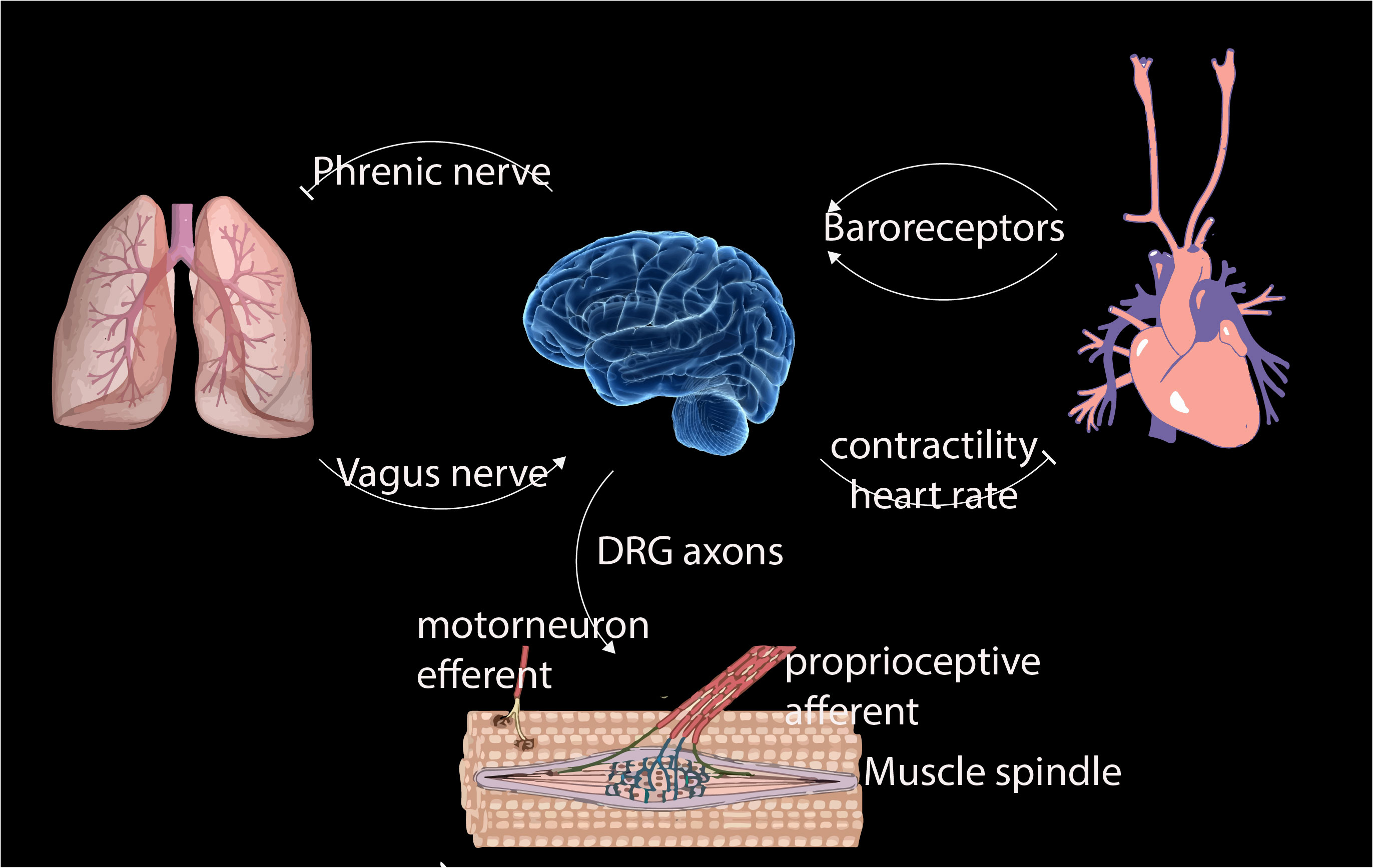
- Krieg, M et al. Mechanical Body-Brain Interactions in C elegans. Current Opinion in Neurobiology, 2022, pdf
- Porta-de-la-Riva, M. et al. Deploying photon for communication within neuronal networks. BioRxiv, 2021 BioRxiv
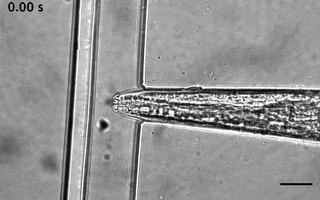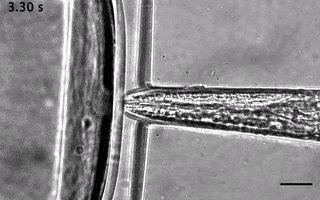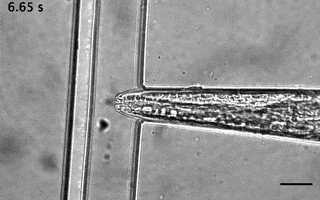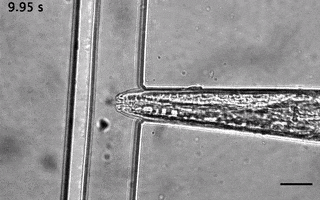
Catala-Castro, F. et al. Direct force measurements of subcellular mechanics in confinement using optical tweezers, JoVE, 2021, pdf
- a) Das, R. et al. An asymmetric mechanical code ciphers curvature dependent proprioceptor activity, Science Advances, 2021 pdf
b) Mechanical Stretch Inhibition Sensitizes Proprioceptors to Compressive Stresses, bioRxiv, 2021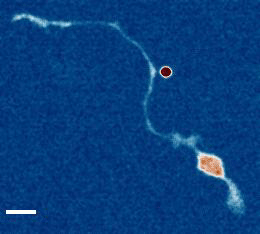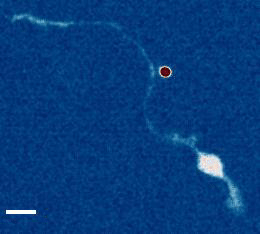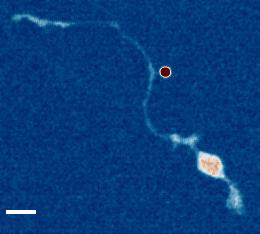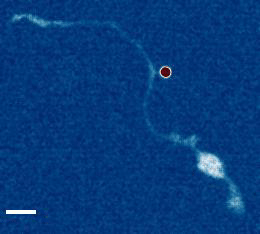
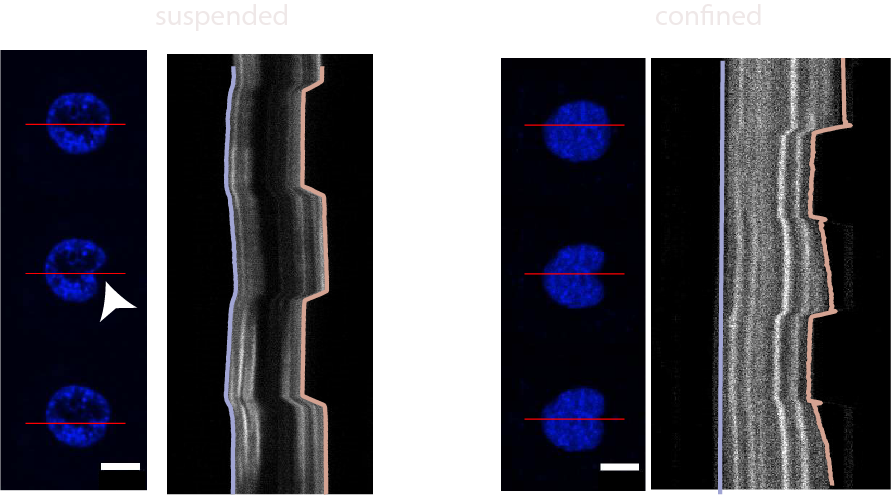
- Venturini et al. The nucleus measures shape deformation for cellular proprioception and regulates adaptive morphodynamics; Science, 370, 2020 Venturini
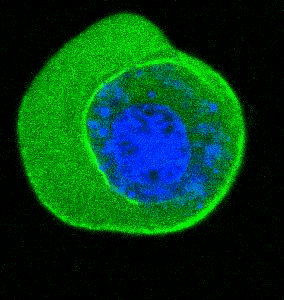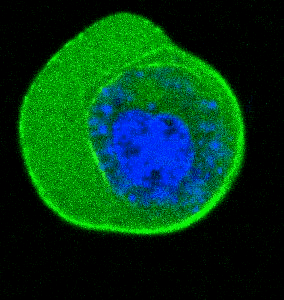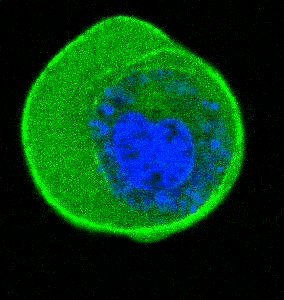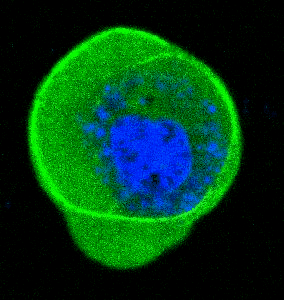
- He et al. Cortical anchoring of the microtubule cytoskeleton is essential for neuron polarity; eLife, 2020.pdf
- He et al. Direction Selectivity in Drosophila Proprioceptors Requires the Mechanosensory Channel Tmc, Current Biology, 2019, 29, 1–12 pdf
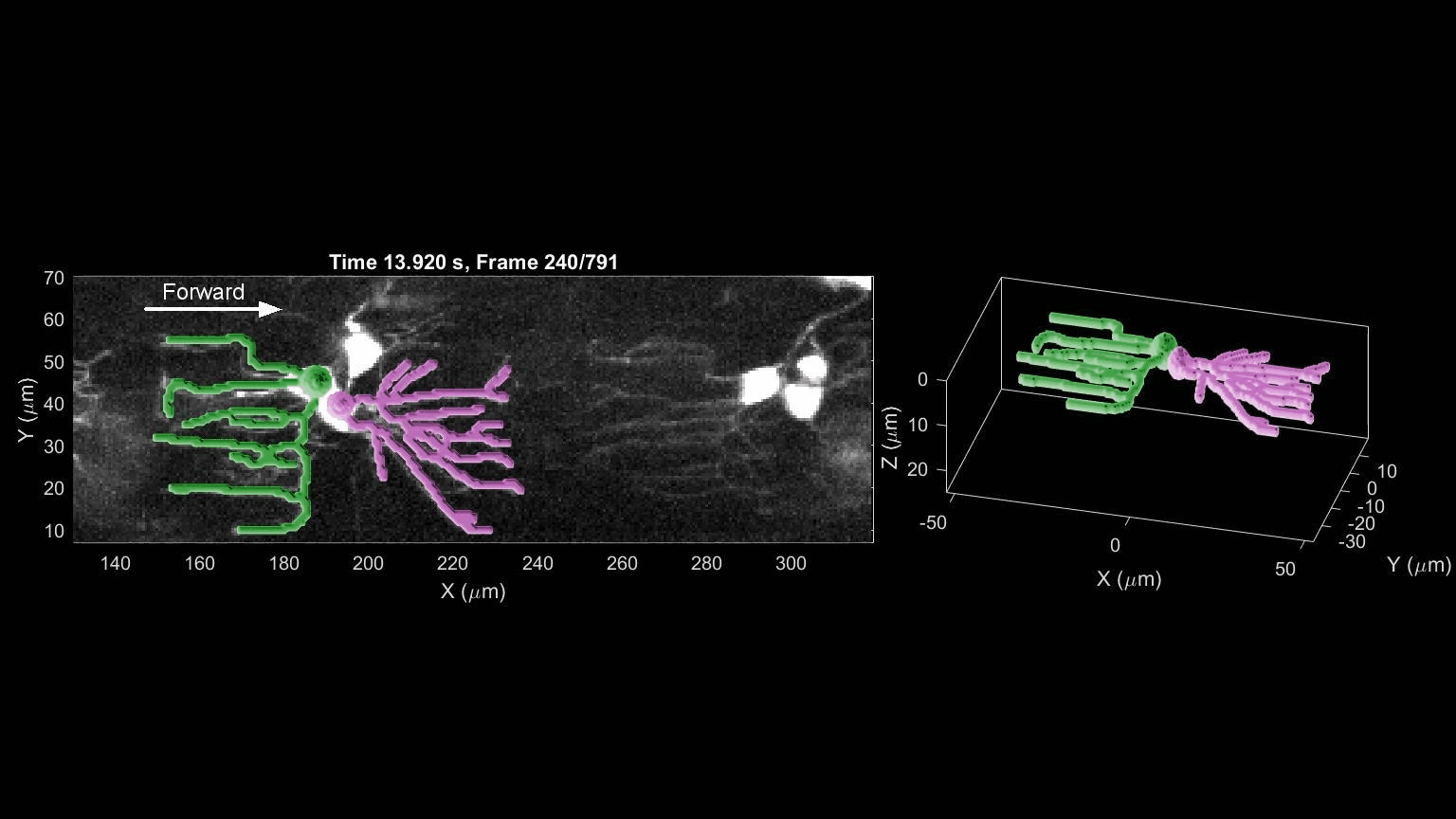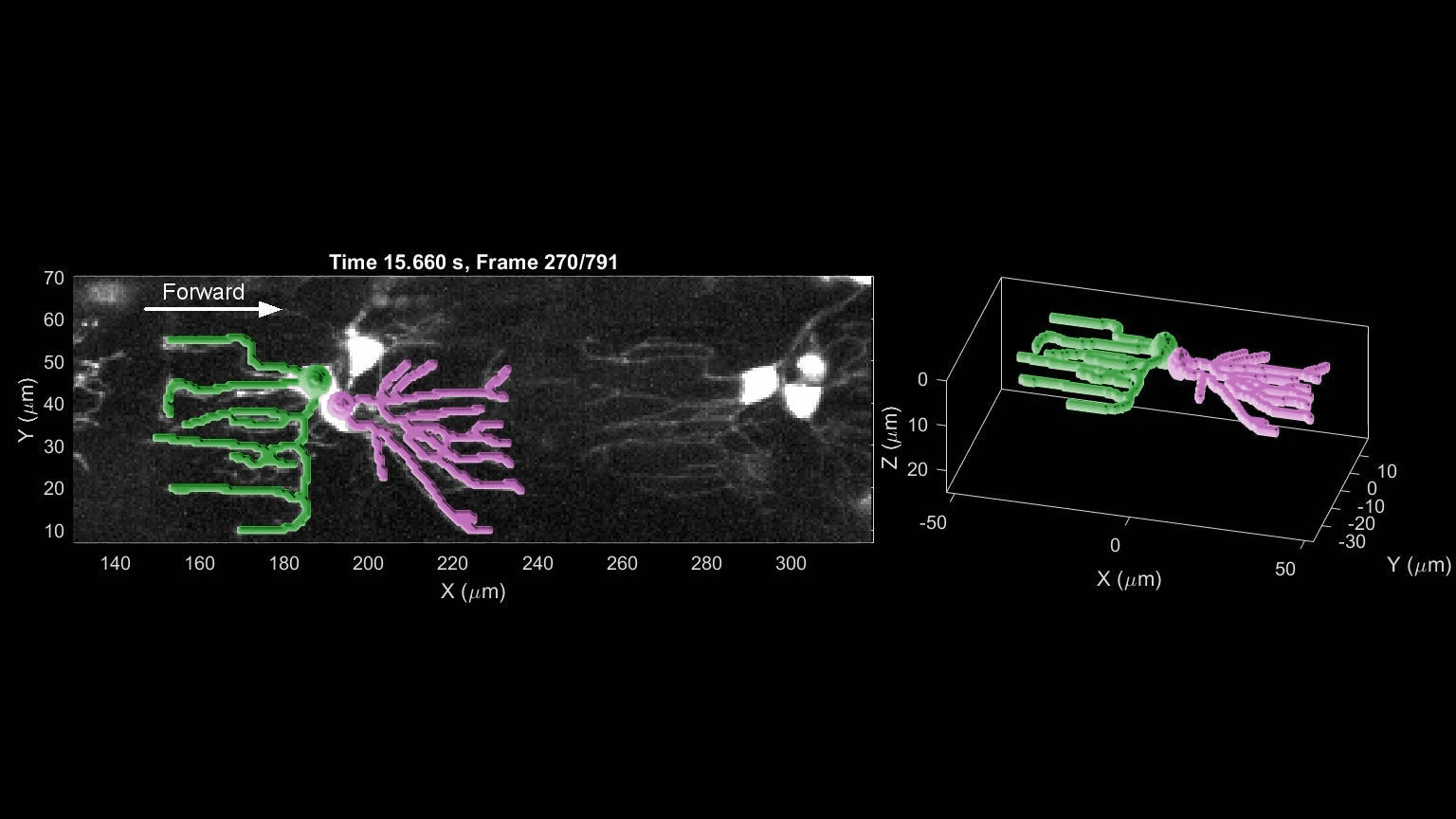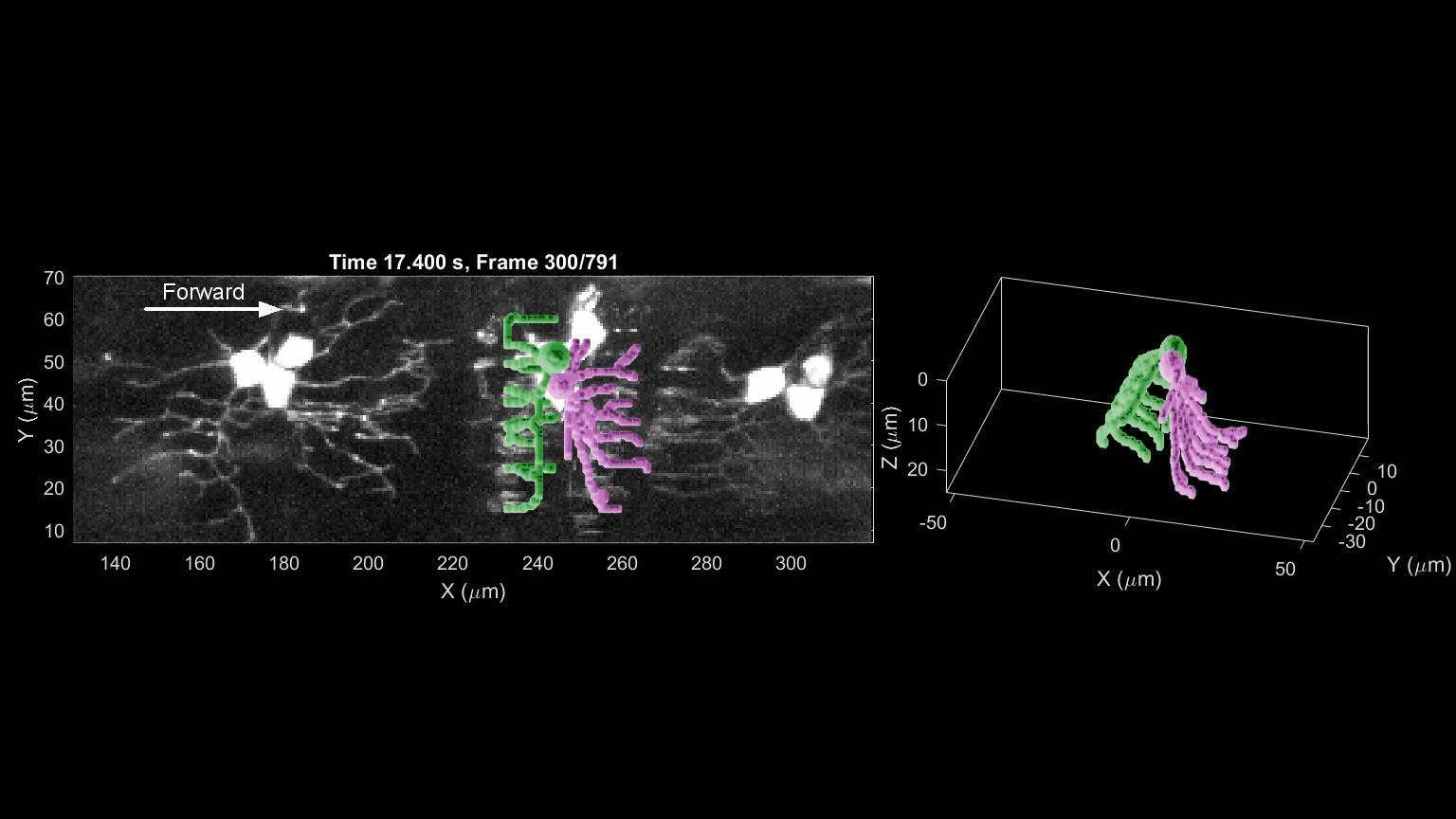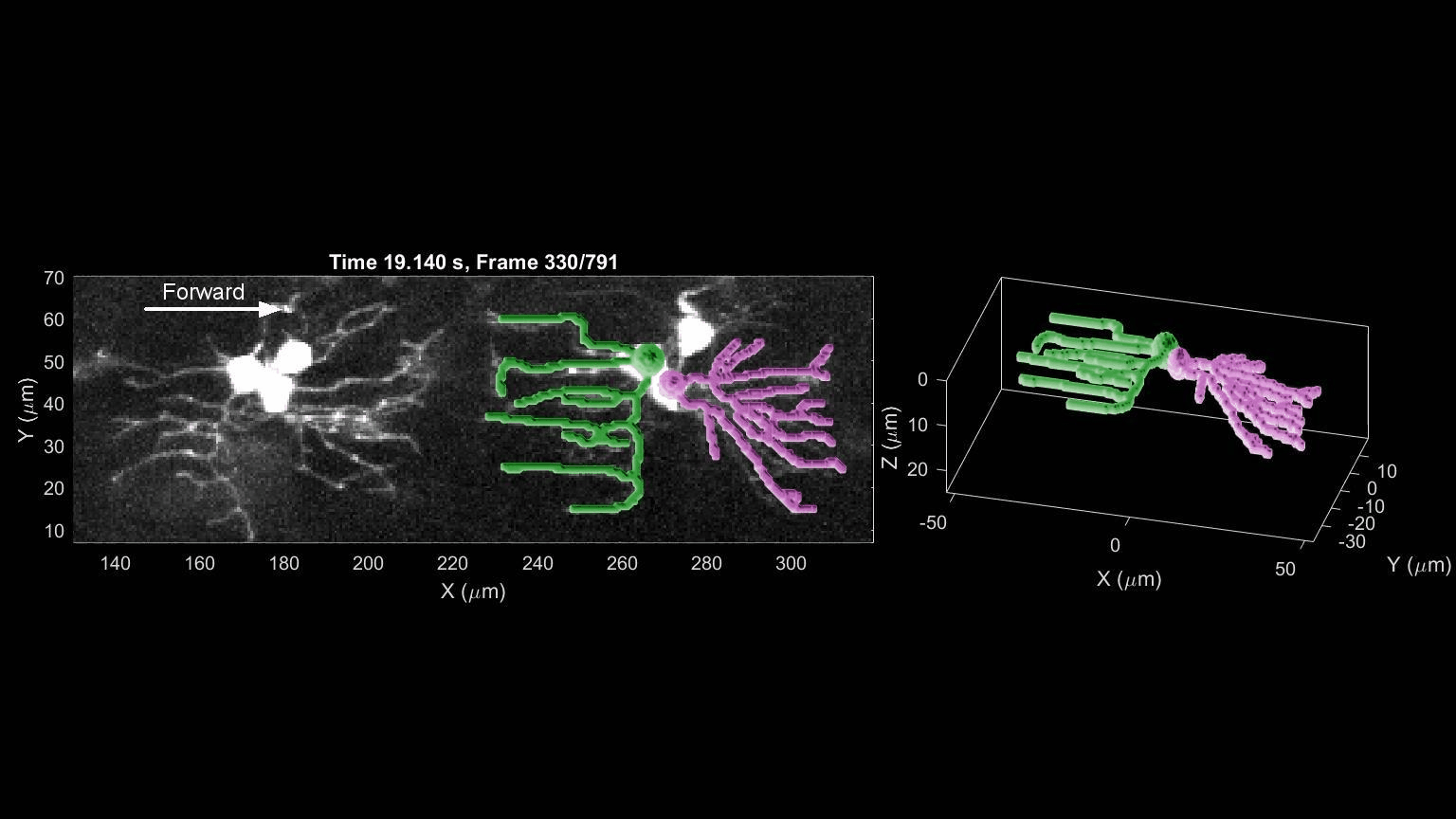
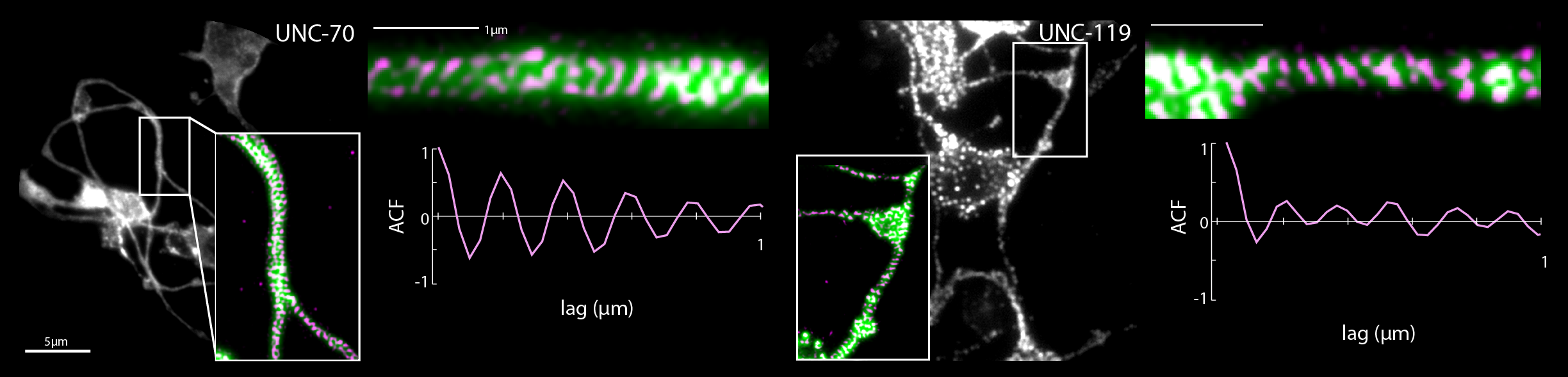
- R. Das, S Wieser, M Krieg, Neuronal stretch reception – making sense of the mechanosense, Experimental Cell Research – special issue Mechanotransduction, pdf
- G Cabré, A Garrido-Charles, M Moreno, M Bosch, M Porta de la Riva, M Krieg, M Gascón-Moya, N Camarero, R Gelabert, J M. Lluch, F Busqué, J Hernando, P Gorostiza, R Alibés Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation Nature Communications, (2019) 10:907 pdf
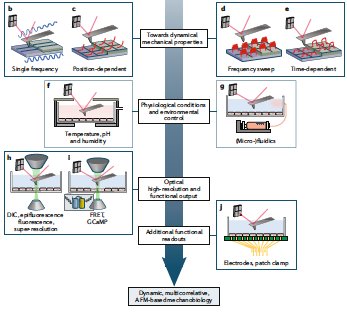
- M Krieg, G. Fläschner, D. Alsteens, B. M. Gaub, W.H. Roos, G. J. L. Wuite, H. E. Gaub, C. Gerber, Y. F. Dufrene, D.J. Muller Atomic force microscopy-based mechanobiology Nature Reviews Physics, 1, 2019 Cover pdf
- H. Fehlauer, A Nekimken, A Kim, B.L. Pruitt‡, M.B. Goodman‡, M.Krieg‡ Using a Microfluidics Device for Mechanical Stimulation and High Resolution Imaging of C. elegans. J Vis Exp. 2018 Feb 19;(132). doi: 10.3791/56530 pdf
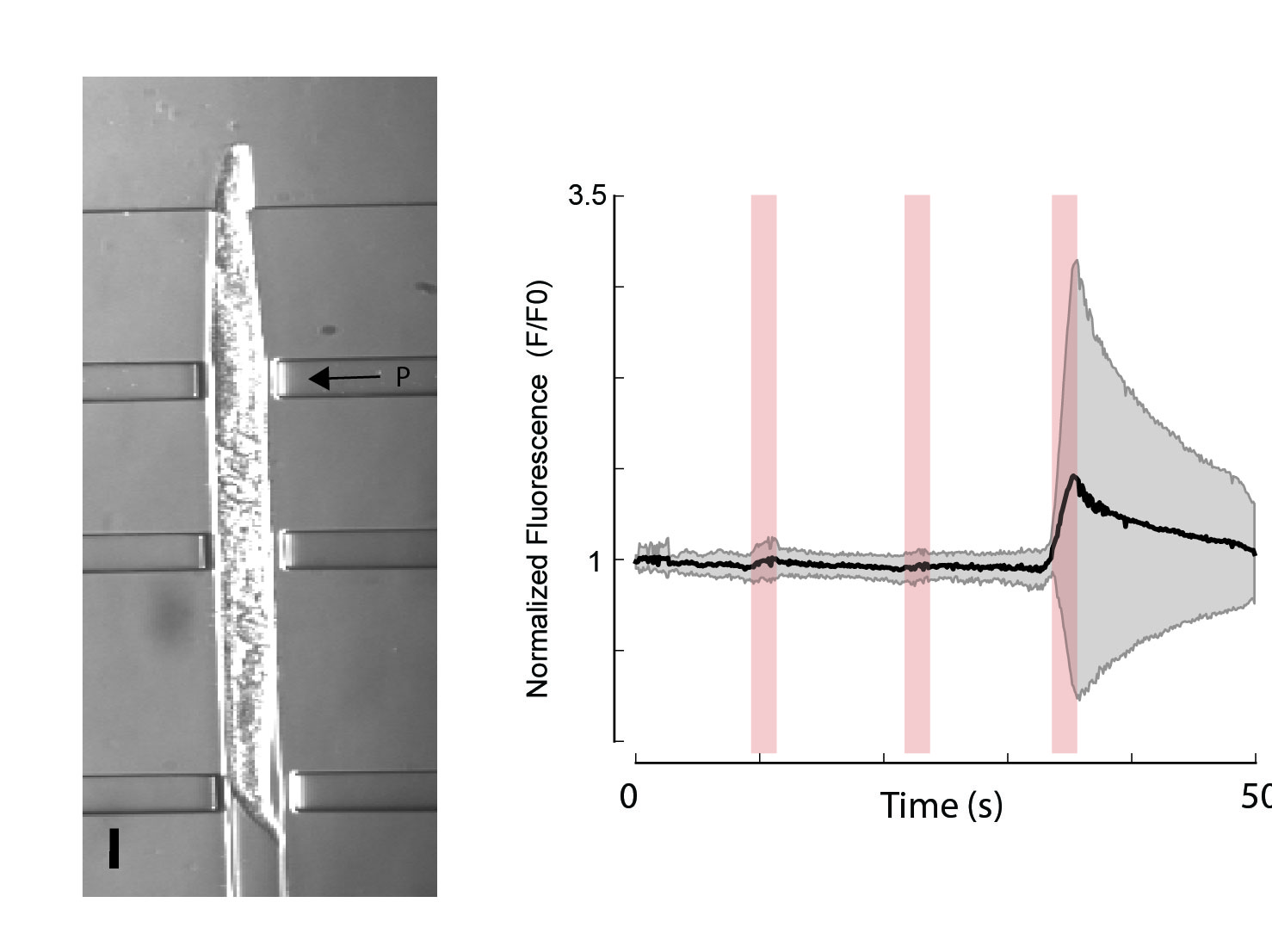
- A Nekimken, H. Fehlauer, A Kim, S.N. Manosalvas-Kjono, P. Ladpli, F. Menon, D. Gopisetty, V Sanchez, M.B. Goodman‡, B.L. Pruitt‡, M.Krieg‡ Pneumatic stimulation of C. elegans mechanoreceptor neurons in a microfluidic trap Lab-on-a-Chip 17, 1116?1127, 2017 ‡ corresponding author pdf
- M.Krieg, J. Stuehmer, J.C. Cueva, R. Fetter, K. Spilker, D. Cremers, K. Shen, A.R. Dunn, M.B. Goodman Genetic Defects in -spectrin and tau Sensitize C. elegans Axons to Movement-induced Damage via Torque-tension Coupling eLife, 6 (2010), e20172 2017 pdf
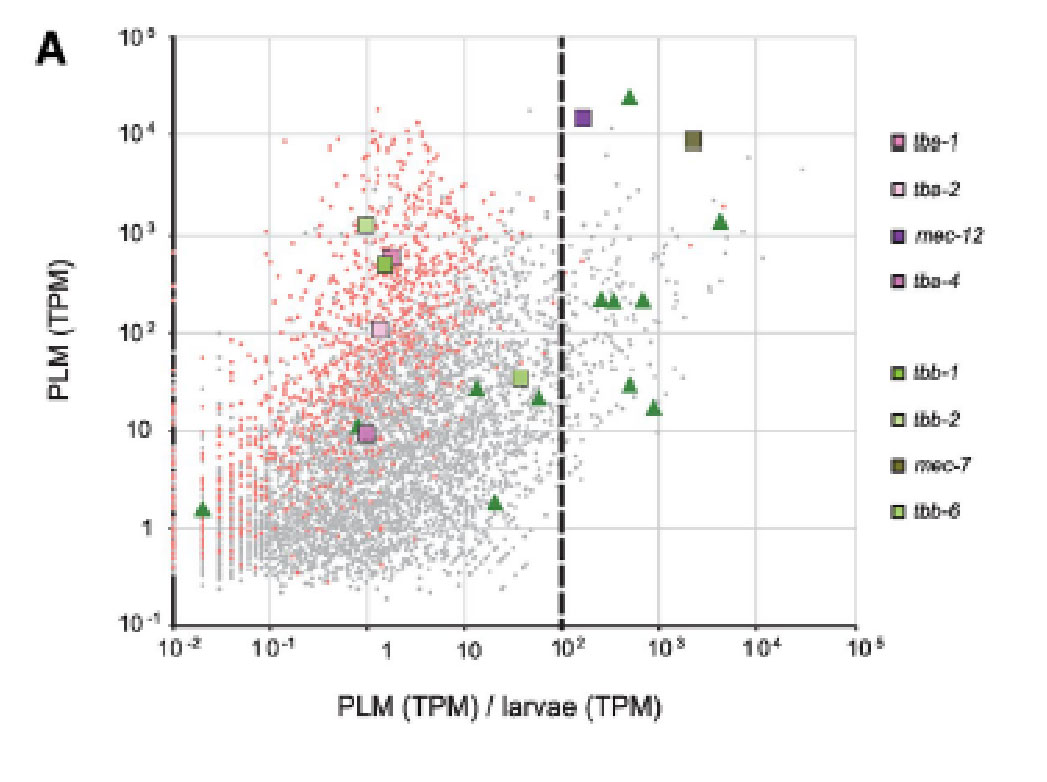
- D Lockhead, E.M. Schwarz, R. O’Hagan, S. Bellotti, M.Krieg, M.M. Barr, A.R. Dunn, P.W. Sternberg, M.B.Goodman. The tubulin repertoire of C. elegans sensory neurons and its context-dependent role in process outgrowth Molecular Biology of the Cell; 2016 pdf
- S. Katta§, M.Krieg§, M.B.Goodman. Feeling force: Physical and Physiological Principles Enabling Sensory Mechanotransduction Annual Review Cell and Developmental Biology, 31, 347-371 2015 § shared first author pdf
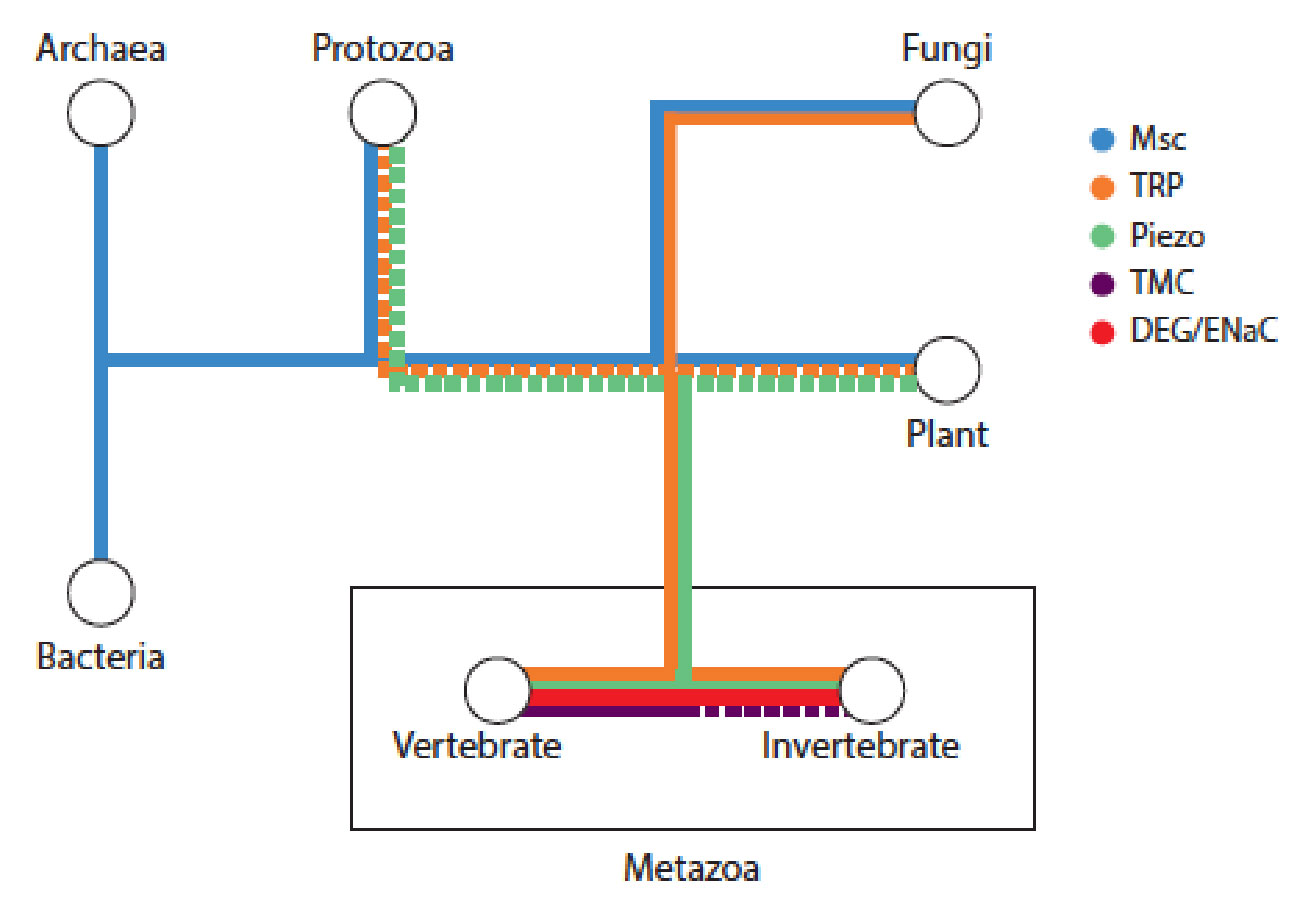
- J. Chai, A. Hamilton, M. Krieg, CD. Buckley, IH Riedel-Kruse, A Force Balance explains local and global cell movements during early zebrafish development. Biophysical Journal 2015 (109) 407-414. pdf
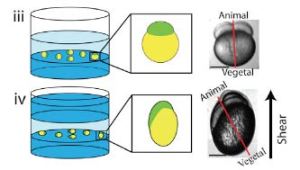
- M. Kelley§, J. Yochem§, M. Krieg§, A. Calixto, M. Chalfie, M. B. Goodman, A. Kuzmanov, M. Heiman, S. Shaham, A. Frand, V. Meli, D. S. Fay A fibrillin-like protein in C. elegans is required for resistance to biomechanical forces during embryonic development elife, 2015; § shared first author pdf
- M. Krieg, A.R. Dunn, M.B. Goodman Mechanical Systems Biology of C elegans touch sensation BioEssays, 2015 Mar;37(3):335-44. doi: 10.1002/bies.201400154. corresponding author
pdf
Here, we review empirical and theoretical studies of how the extracellular matrix (ECM), cytoskeleton and the plasma membrane transmit mechanical force, and discuss how these concepts apply to the sense of touch.
- R. Schubert, N. Strohmeyer, M. Bharadwaj, S. P. Ramanathan, M. Krieg, J. Friedrichs, C. M. Franz, D. J. Muller Assay for characterizing the recovery of vertebrate cells for adhesion measurements by single-cell force spectroscopy. FEBS Letters, 2014 Oct 1;588(19):3639-48. doi: 10.1016/j.febslet.2014.06.012. pdf
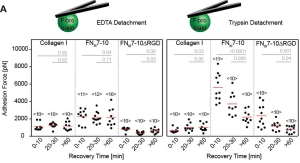
We always left cells to recover before adhesion measurements with the AFM. Here is why. Rajib and Nico characterized the recovery time of fibroblast and HeLa cells after dissociation from their culture dish.
- M. Krieg, A. R. Dunn, M. B. Goodman. β-Spectrin and the Mechanical Control of the Sense of Touch. Nat Cell Biol, 16(3), 22433. doi:10.1038/ncb2915, 2014 Cover pdf

Surprise: Spectrin is important. Featured by NIH: http://www.ninds.nih.gov/news_and_events/news_articles/news_article_touch_05162014.htm
- V. Vazques, M. Krieg, D. Lockhead, M. B. Goodman. Polyunsaturated Fatty Acid-Containing
Phospholipids Enhance Neuronal Cell Mechanics and Touch in C. elegans. Cell Reports, doi:10.1016/j.celrep.2013.12.012, 2013 pdf
 In essence, physical properties of lipid bilayers are crucial for ion channel function.
In essence, physical properties of lipid bilayers are crucial for ion channel function.
- B. Strilic, J. Eglinger, M. Krieg, P. Babal, D. J. Müller, E. Lammert. Blood vessel lumen
formation via electrostatic repulsion. Curr Biol, 20(22):2003–2009, 2010 pdf
Cells repel each other using a negatively charged surface coat to make space for interstitial fluid. - A. Diz-Munoz§, M. Krieg§,‡, M. Bergert, I. Ibarlucea-Benitez, D. Müller, E. Paluch‡, and C. P. Heisenberg‡. Membrane-to-cortex attachment controls bleb formation and migration in germlayer progenitors. PLOS Biol, 8(11): e100054, 2010 § shared first author, ‡ corresponding
author pdf
As simple as this: Membrane tension influence migration by tuning formation of cell protrusion. Lowering membrane tension by knocking down membrane-csk attachment proteins such as ezrin or myosin 1, causes abberant blebbing and slower movement of the perturbed cells.
- M. Köppen, P. Oteiza, M. Krieg, S. Preibisch, C. Farias, C. Melo, E. Pulgar, D. Müller, M. Tada, S. Hartel, C. P. Heisenberg and M. L. Concha. Planar Cell Polarity signalling coordinates epithelial organ formation by regulating cell adhesion properties in progenitor cells. Development, 137(20):3459–3469, 2010. pdf
Kupffers vesicle determines left-right asymmetry in developing zebrafish embryos. We used fluorescent activated cell sorting to sort gfp-expressing forerunner cells, progenitors of the KV, and measure their adhesive properties to one another. A reduction in adhesion correlates with perturbed KV function and defects in heart development. - Y. Arboleda-Estudillo, M. Krieg, J. Stuhmer, N. A. Licata, D. J. Muller, and C. P. Heisenberg. Movement directionality in collective migration of germ layer progenitors. Curr Biol, 20(2):161–169, 2010. pdf
How does migration of embryonic progenitor cells in 3D depend on adhesive properties? We used SCFS to measure the adhesive force and show that a reduction in adhesion correlates with a loss of movement directionality of developing mesodermal cells. We propose that mesendodermal cells migrate as a collective and that E-cadherin is needed to `feel’ neighbouring cells.
- D. J. Muller, M. Krieg, D. Alsteens, and Y. F. Dufrene. New frontiers in atomic force microscopy: analyzing interactions from single-molecules to cells. Curr Opin Biotechnol, 20(1):4–13, 2009.pdf
Yet another review article about SCFS in cell biology.
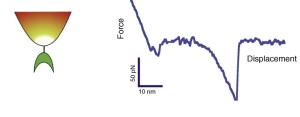
- M. Krieg, J. Helenius, C. P. Heisenberg, and D. J. Muller. A bond for a lifetime: employing membrane nanotubes from living cells to determine receptor-ligand kinetics. Angew Chem Int Ed Engl, 7(50):9775–7, 2008. pdf
Here we used membrane tethers pulled from living cells to measure binding kinetics of mannose-ConA bond. Due to membrane tension, a constant force acts on the bond that couples the cell membrane to the tip of an AFM cantilever. By modulating the membrane tension, and varying the pulling speed, we could adjust the force on this bond. The unbinding kinetics nicely followed the Bell model of force accelerated bond dissociation. - M. Krieg, Y. Arboleda-Estudillo, P. H. Puech, J. Kafer, F. Graner, D. J. Muller, and C. P. Heisenberg. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol, 10(4):429–36, 2008. Cover, news&views pdf
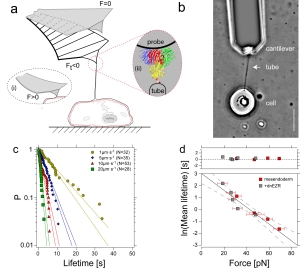
We used biochemical and biophysical tools to understand the role of adhesive and mechanical forces during germlayer progenitor sorting in zebrafish. Specifically, we found that hierarchiy in cortex tension predicts the spatial organization of organ progenitor during gastrulation.
- F. Ulrich§, M. Krieg§, E. M. Schotz§, V. Link, I. Castanon, V. Schnabel, A. Taubenberger, D. Muller, P. H. Puech, and C. P. Heisenberg. Wnt11 functions in gastrulation by controlling cell cohesion through rab5c and e-cadherin. Dev Cell, 9(4):555–64, 2005. § shared first author pdf
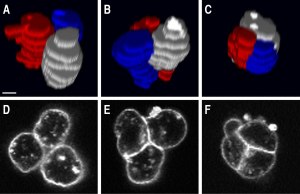
Here we showed that wnt11, a signaling molecule of the non-canonical wnt pathway regulates adhesion to e-cadherin by rab-5 mediated endocytosis. The featured image shows sequential images of a cohering cell-triplet. Using SCFS, we measured cadherin-mediated adhesion down to the level of a single molecule. The article was featured in Nature Reviews MCB, Vol. 6 No 11, 2005
- P. H. Puech, A. Taubenberger, F. Ulrich, M. Krieg, D. J. Muller, and C. P. Heisenberg. Measuring cell adhesion forces of primary gastrulating cells from zebrafish using atomic force microscopy. J Cell Sci, 118(Pt 18):4199–206, 2005. pdf
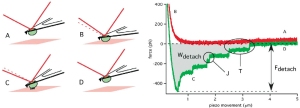 The first application of single cell force spectroscopy in the Muller Lab. What a start of a glorious time! We could show that the non-canonical wnt pathway influence cell adhesion to fibronectin – a surprising finding that was a featured article of the JCS issue. featured-pdf
The first application of single cell force spectroscopy in the Muller Lab. What a start of a glorious time! We could show that the non-canonical wnt pathway influence cell adhesion to fibronectin – a surprising finding that was a featured article of the JCS issue. featured-pdf - A. Kedrov, M. Krieg, C. Ziegler, W. Kuhlbrandt, and D. J. Muller. Locating ligand binding and activation of a single antiporter. EMBO Rep, 6(7):668–74, 2005. pdf
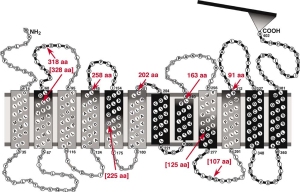
Here we used Atomic Force Microscopy based single molecule force spectroscopy to measure the forces that stabilize the ligand binding site of a sodium-proton ion channel.