Microfluidics is like plumbing for really tiny things, like drops of water or even smaller. Imagine you have a system of tiny channels or pipes, like the ones in your home, but these are so small that they can carry incredibly tiny amounts of liquids, like a single drop of blood or a speck of chemicals.
Scientists and engineers use microfluidics to manipulate these tiny amounts of liquids with great precision. They can mix different liquids together, separate them, or even perform experiments on a super tiny scale. It’s like having a mini laboratory that works with microscopically small volumes of fluids, and it’s used in various fields, from medical diagnostics to making small electronic devices.
Typically, fluids are moved and processed in ultra-small capillaries in chips casted from PDMS, a chemically inert elastomer. Due to the size of the worm, µFluidic technology is very popular for single animal handling and manipulation. Over the last few years, we have combined microfluidic trapping of single animals with fast imaging of neuronal activity under various external stimuli. Don’t hesitate to reach out in case you are interested in using, building or collaborating on any of these devices, or if you have some new chip ideas that you want to bounce around.
1. The body wall chip (Nekimken, LabChip, 2017).
Our most popular devices enable high resolution imaging of living worms, while presenting them with a mechanical stimulus. The idea is to create a high-throughput platform to probe worm mechanics and to test the effect of mechanical force during physiology and ageing. In agreement with previous data we found that touch receptor neurons are highly sensitive to vibrations, similar to the tactile sensors in your finger tips. The device comes now in various modifications, e.g. to host males and decipher sexually dimorphic neuronal circuit and their response to. mechanical stresses (Setty, Nat Comms, 2022). It has also been used to visualize force transmission in touch receptor neurons using a MEC-2 tension sensor derived from TSMod (Sanfeliu, Nature Cell Biology, 2023). This movie shows the mechanical stimulation of a single worm immobilized in a horizontal channel. As the pressure is delivered to the animals, its touch receptor neuron lights up as a consequence of neuronal activation.
This movie shows the mechanical stimulation of a single worm immobilized in a horizontal channel. As the pressure is delivered to the animals, its touch receptor neuron lights up as a consequence of neuronal activation.
2. The TRAP’N’SLAP device (Porta, Nature Methods, 2023).
We have ever since adapted the body wall touch design in a device that presents a mechanical stimulus to the nose of the worm. As with the other device, the socalled TRAP’N’SLAP device is compatible with high resolution microscopy to image the consequence of the mechanical stimulus on neuronal activity. It also comes in two versions for hermaphrodites and males.
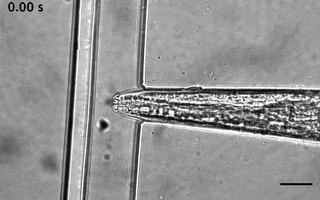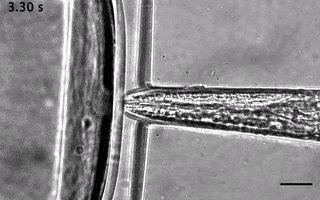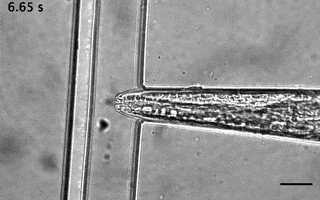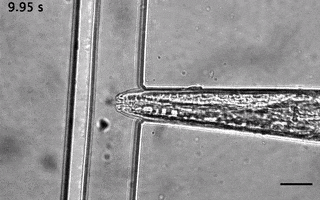
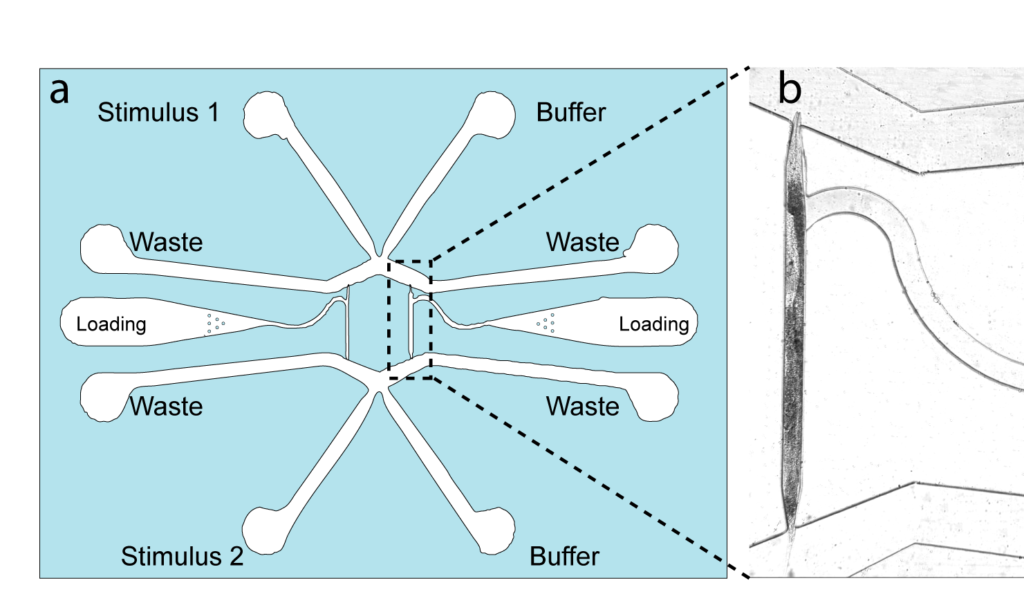
3. The dual olfactory device (Gat, Cell Reports, 2023; Karimi, APL BioEngineering, accepted).
Our exploration into olfactory science. We designed a device that facilitated the automated and simultaneous delivery of an olfactory, chemical stimulus to senseory neurons located either in the head, tail or both.
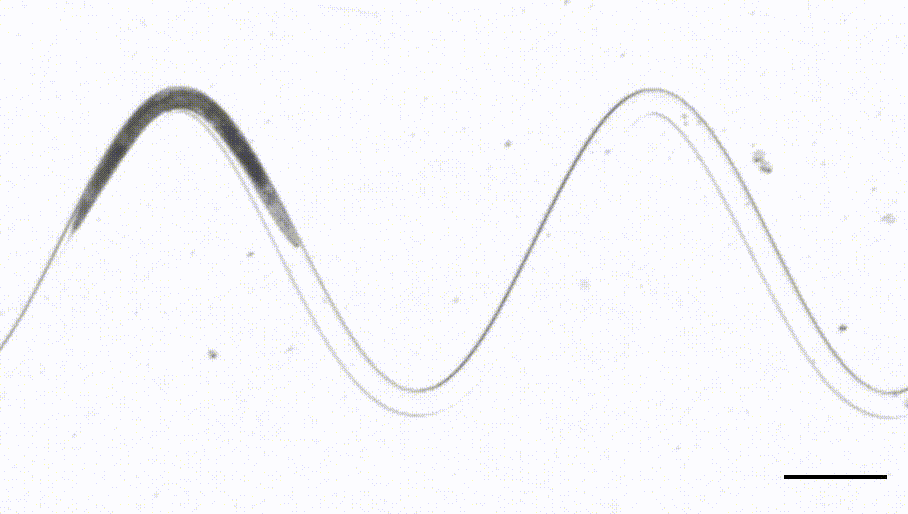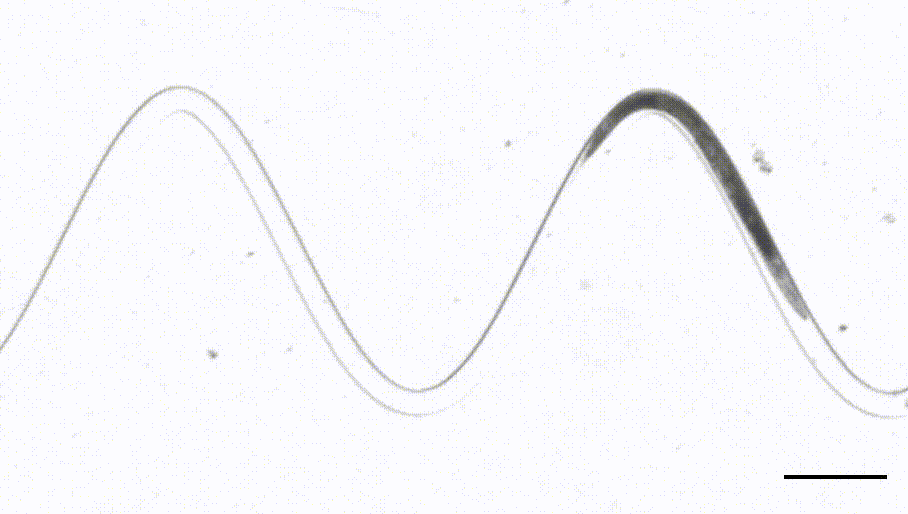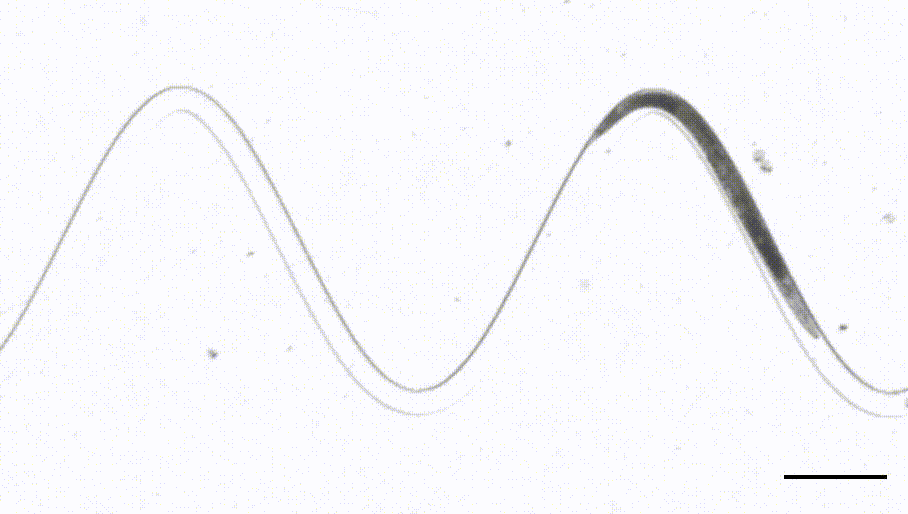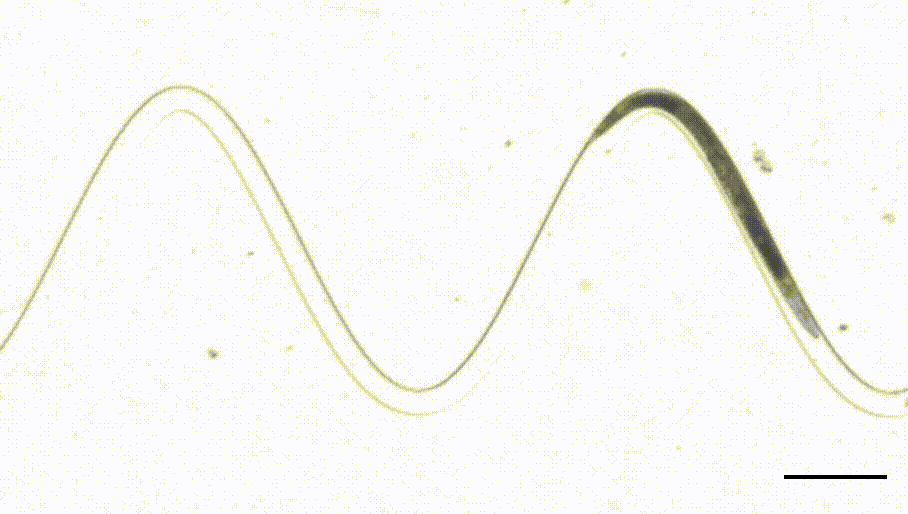
4. Waveform sampler. (Das et al, Science Advances, 2021)
This is a new adaptation of the artificial dirt and waveform sampler introduced originally by Shawn Lockery in 2008. Leveraging a device that sports channels with different curvatures, we were able to perform FRET-force microscopy in a b-spectrin tension sensor of animals in different curvature and calcium imaging on a neuron that responds to proprioceptive curvature cues.