FLint – Guided Transgene integration using Fluorescence landmark interference
by Nawaphat Malaiwong
We piggy backed on a library of ~300 transgenic animals carrying a single copy insertion of a gene encoding a fluorescent protein. This provides a safe landing site guided by visual screening of the successful integrants.
Check back for updates or under under this link: FLINT
Introduction
The protocol describes how to use the Fluorescent Landmark Interference: FLInt method to integrated transgene in C. elegans, from the selection of background strains, preparation of injection mix, and animal screening. The procedure follows the principle of the site-target DNA excise via CRISPR/Cas9 at the fluorescent marker sites described in our publication (Malaiwong et al., 2023). The FLInt protocol for integration covers the establishment of the transgenic C. elegans from (1) the newly forming extrachromosomal array, (2) the existing array, and (3) the integrated Cas9 gene.
Materials
Worm strains and bacteria
EG7835 [oxTi556 I (eft-3p::tdTomato::H2B)]
EG7866 [oxTi564 II (eft-3p::tdTomato::H2B)]
EG7898 [oxTi619 III (eft-3p::tdTomato::H2B)]
EG7911 [oxTi705 IV (eft-3p::tdTomato::H2B)]
EG7944 [oxTi553 V (eft-3p::tdTomato::H2B)]
EG7989 [oxTi668 X (eft-3p::tdTomato::H2B)]
EG8888 [oxTi936 X (eft-3p::gfp::NLS)]
EG8958 [oxTi1022 I (eft-3p::gfp::NLS)]
MSB1247 unc-119(ed3) III; oxTi553 (eft-3p::tdTomato::H2b) V; oxSi1091(mex-5p::Cas9(smu-2 introns) + unc-119(+)) II
E. coli (OP50)
all tdTomato EGxxx strains bear unc-119(ed3) mutation and the Cbr-unc-119 rescue construct as specified on wormbuilder.org
Reagents
- Plasmids
- plasmid of interest
- co-injection marker plasmid, myo-2p::mCherry, myo-3p::mCherry, unc-122p::GFP
- CRISPR reagents
- crRNA against tdTomato (5´- 64 GTGATGAACTTCGAGGACGG|CGG-3’)
- crRNAs against GFP (5´-CTTGTCACTACTTTCTGTTA-3´) and (5´-TGAACTATACAAATGCCCGG-3´)
- tracrRNA
- Cas9 nuclease
- Chemicals
- DNA ladder (invitrogen)
- Chemicals for preparing NGM plates
- Halocarbon oil
- Agarose
Equipment
- 5-cm petri dish
- 3-cm petri dish
- Coverslip
- Painting brush
- Fluorescent stereoscope
- Microinjector apparatus
- Needle puller
- Loading pipette tip
- Glass capillary
- Worm picker (made from the platinum wire)
- 20C incubator
- 25C incubator
- Thermoblock
Integration of new forming array by injecting RNP complex
Transgenic worm selection (1-2 weeks before integration)
- The selection of background strains for integration depends on the desired landing site for the transgene of interest. Based on the data from the article, the landing site at the center of each chromosome are recommended based on the high effeciency and success.
- Integration on Chromosome I: use EGxxx
- Integration on Chromosome II
- Integration on Chromosome III
- Integration on Chromosome IV
- Integration on Chromosome V
- Integration on Chromosome X
- The parental strains should be cultured at 20C in order to prevent the systemic defects caused by the tdTomato gene. In addition, non-contaminated OP50 is preferable.
Preparation of CRISPR mix (approx. 30 min)
(The concentration of crRNA in this protocol has been adjusted from the protocol showed in the article)
Prepare the stock solution of crRNA and tracrRNA to obtain 167 mM by adding milliQ water
Mix 1 uL of crRNA and 1 uL of tracrRNA in a 1.5 mL tube
Adjust the volume up to 9 uL by milliQ water
Incubate the solution at 95C for 5 min using the Thermoblock
Incubate the solution at RT for 5 min
Add 1 uL of Cas9 nuclease
Aliquot the mix into the PCR tube (2 uL each)
Store at -20C for the further use
Preparation of injection mix (10 uL)
Mix the plasmid of interest and co-injection marker to obtain the desired concentration
Add the DNA ladder (initial conc. = 500 ng/uL) to obtain 100 ng/uL of total DNA
Add milliQ water up to 8 uL
Add the CRISPR mix (in the PCR tube described above) (total vol. = 10 uL)
Spin the mix using the centrifuge at the high speed for 8-10 min to prevent the debris in the microneedle
Transgene integration
Day 1: microinjection (1-3 hours)
- Preparation of the injection pad (1-3 days before microinjection day)
- Melt the 1.5-2% agarose in milliQ water
- Drop the melted agarose onto the center of the coverslip (size=)
- Put another coverslip on top until the agarose cool down and solidify
- Remove one of the coverslip
- Dry the agarose pad (on the coverslip) at room temperature overnight
- Keep the pad in the clean and dry place
- Preparation of injection mix and microneedle
- Prepare the microneedle by pulling the cappillary using the needle puller
- Pipette 2-5 uL injection mix (described above) using the loading tip
- Load the mix into the needle
- Setup the microneedle into the microinjector apparatus
- Preparation of worms
- Select 20-30 young, healthy adult worms from the culture plate using painting brush and halocarbon oil
- Place the worms onto the NGM plate without OP50 (30-min starvation before injection is optional, it facilitates visualizing gonads with less intestinal contents)
- Transfer worms to the injection pad using painting brush with halocarbon oil
- Flatten worms on the injection pad by completyly attach to the injection pad (worms will not move)
- Place the injection pad on the microinjector stage
- Adjust the focal plane of the microneedle and worm’s gonad with 40X objective lens view
- Move the microneedle by the micromanipulator against the worm or move the stage against the needle
- Penetrate the needle into the worm when the needle and the gonad arm are in the same plane
- Apply the air pressure to push the solution into the gonad (the successful injection can be observed by the movement of the syncytial nuclei toward the solution flow)
- Inject in both gonad arms of each worm
- Recover the injected worms by dropping M9 buffer into the injection pad (worms will be rehydrated, detach from the pad, and start moving)
- Transfer 10 injected worms into the 5-cm NGM plate with OP50 (20-30 worms are recommended for each integration)
- Incubate at 25C for 3 days
- Day 3: F1 screening and isolation (1 hour)
- The positive F1 will be obtained by the following steps:
- Screen for the worms that lost the tdTomato signals but express the co-injection marker protein from the plate
- Single them out into the 3-cm petri dish with NGM/OP50
- Note the number of transgenic lines (= number of positive F1)
- Culture F1 in 25C incubator
- Day 6: F2 screening and isolation (2-3 hour)
- From each F1 plate, screen for the F2(s) with high transmission frequency (high proportion of co-injection marker expression worms)
- Discard the plate without extrachromosomal array transmission or less than 50%
- Collect the plate with approx. 75% transmission frequency
- From each plate, single out six positive F2 into new 3-cm petri dish with NGM/OP50
- Label each six plates(F3) from the same origin(F2) as the same transgenic line
- Culture F2 in 25C incubator
- Day 9: F3 screening and calculating the integration efficiency (1 hour)
- Screen all plates from day 6 using the fluorescent stereoscope
- Search for the 100% transgenic F3 progenies from each line (6 plates)
- Calculate the integration efficiency by (no. of integrated line / no. of positive F1) x 100
- The integrated lines can then be used for experiment
Integration of new forming array using integrated Cas9
- To reduce the cost of gene editing reagents, using the genetically-encode protein or RNA became the reliable option. Here, we adapted the C. elegans strain, EG9615, expressing Cas9 protein in the germ line with the optimizing intron for the robust expression and efficiency (ref.) With such background P0 animal, we injected the CRISPR mix which made by the crRNA and tracrRNA and proceed the screening step as previously described. This strategy can eliminate the use of purified Cas9 from purchasing, also, we could sustainably obtain such protein in the worms in all generations. However, the integrated Cas9 is not preferable for the further use. After obtaining the candidate, the integrated Cas9 allele (oxSi1091[Pmex-5::cas9(+smu-2 introns)::tbb-2 3’UTR unc-119+] II) should be discarded from the integrated array by outcrossing with N2.
- The procedure of worm selection, microinjection, and screening are similar to the previous method. However, the preparation of the CRISPR mix is different.
Preparation of CRISPR mix for integrated Cas9
- Prepare the stock solution of crRNA and tracrRNA to obtain 167 mM by adding milliQ water
- Mix 1 uL of crRNA and 1 uL of tracrRNA in a 1.5 mL tube
- Adjust the volume up to 10 uL by milliQ water
- Incubate the solution at 95C for 5 min using the Thermoblock
- Incubate the solution at RT for 5 min
- Aliquot the mix into the PCR tube (2 uL each)
- Store at -20C for the further use
Integration of existing array
With the variation and inconsistency of gene expression of an array, the preliminary observation of expression pattern is required prior to integration. This protocol is an alternative integration using FLInt for the selected extrachromosomal lines.
Generation of extrachromosomal array
- Select the plasmid for injection or co-injection plasmid with Amp resistance gene
- Prepare the injection mix by adding plasmid of interest, co-injection marker plasmid, and DNA ladder (100 ng of DNA in total)
- Inject into the P0 strain (such as N2)
- Screen and isolate the F1 progenies with co-injection marker expression
- Observe the gene expression from the transmitted array in F2 progenies
Selection of desired array and cross
- Cross the extrachromosomal transgenic line with the selected tdTomato landing site, for example, the EG7944 for chromosome V integration
Preparation of CRISPR mix
- Prepare the stock solution of crRNAs and tracrRNA to obtain 167 mM by adding milliQ water
- Mix 1 uL of crRNA (tdTomato), 1 uL of crRNA (AmpR) and 2 uL of tracrRNA in a 1.5 mL tube
- Adjust the volume up to 9 uL by milliQ water
- Incubate the solution at 95C for 5 min using the Thermoblock
- Incubate the solution at RT for 5 min
- Add 1 uL of Cas9 nuclease
- Aliquot the mix into the PCR tube (2 uL each)
- Store at -20C for the further use
Integration by microinjection
- Prepare the injection mix by diluting the CRISPR mix (2 uL) with 8 uL miliQ water
- Inject the solution into the gonad of the P0 strain (tdTomato (V) + extrachromosomal array)
- Culture the P0 animals in 25°C
- Screen and single out the positive F1 (co-injection marker positive + non-tdTomato)
- Culture the F1 progenies for 3 days in 25°C
- Screen for the high transmitted array (75%) in F2 population and single out six worms from each selected plate
- Culture the F2 progenies for 3 days in 25°C
Screening for integrants
- Screen for the 100% transgenic animal (F3) with co-injection marker expression
Verification of integrant
- visual screening
The successful transgene integration can be easily observed based on the expression of co-injection marker. Since the integrated array is built up from all inject plasmid DNA, the expression of co-injection marker could refer to the existence of other co-injected DNA. However, the expression pattern of integrated transgene should be clarified in case of the known phenotype such as tissue specific with robust fluorescent signals. The screening of integrated array is usually done with the F3 progenies with 100% transgenic animals obtained from a single F2. The strong fluorescent marker can be observed under the fluorescent steromicroscope unless the integration method was proceeded with the phenotypic marker e.g. antibiotics or defective behaviors, normal stereoscope is feasible for screening procedure. In addition, the array integration without co-injection marker is possible in case the transgene of interest exhibits the distinguishable pattern of expression. There are several ways to determine the expression of the injected or integrated transgenes, however, the principle of such verification is to obtain the 100% transgene in the population.
- PCR
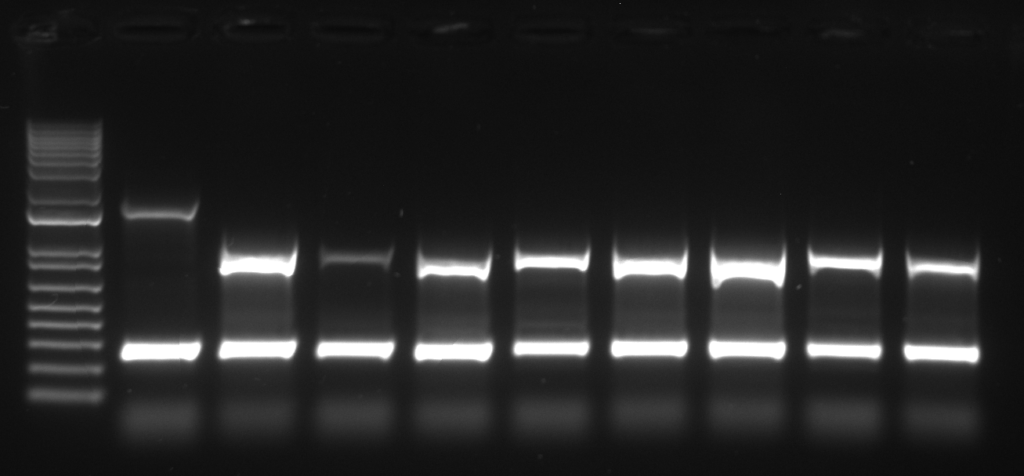
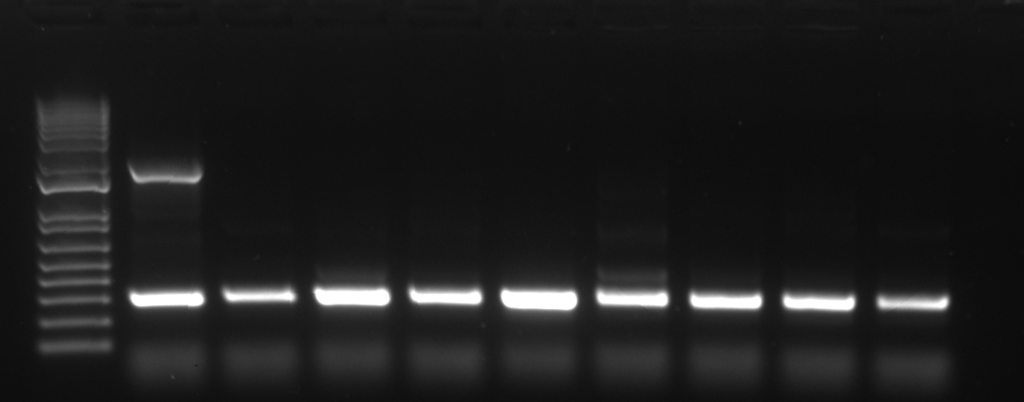
The molecular verification of integrated transgene using PCR is based on the existence of undefined portion of array between tdTomato cutting site. Compared to the self-ligated tdTomato, the integrated gene would not give the amplicon from the PCR reaction. In this protocol, we introduce the 4-primer PCR reaction adjusted from the previous report (Malaiwong et al., 2023) in which 2 region of chromosome would be amplified.
PCR verification can be used when the candidate animals do not express the co-injection marker. There are 2 amplicons from the reaction: (1) the control band (0.3kb), eft-3 promotor, which represent the correct PCR amplification, (2) the excised tdTomato amplicon (0.7 kb) which represent the length of tdTomato landing site. The presence of tdTomato band indicates the non-integrated, the absence of tdTomato band indicates the integrated.
- PCR primers
- Forward1
- ttggtcttttattgtcaacttccattgg
- Reverse1
- ccaacatgattagtcagatgaccaga
- Forward2
- tttataatgaggtcaaacattcagtcccagcgtttt
- Reverse2
- TTACTTGTACAGCTCGTCCATGC
- Preparation for PCR reaction
- Genotyping integrated candidate using PCR can be done following the instruction of particular commercial Kit.
- Instead of using 2 primers, 4 primers are used.
- Annealing temperature = 55C
- Extension time = 40 sec – 1 min
- Amplicon size = 0.3 kb and 0.7 kb
- Genotyping of extrachromosomal array F2
- Genotyping of integrated F2 (homozygous allele)
Limitation and considerations
Incubation time
- This protocol was described the culturing temperature of P0, F1, F2, and F3 at 25°C. However, this procedure cannot be proceeded with the temperature-sensitive phenotype of transgenes (from the genetic background or from the integrated gene). The appropriate temperature need to be considered prior to perform this method. Therefore, the time period of the screening step might be shifted. In addition, culturing P0 in 16°C decreases the efficiency of integration.
Existing array and the false positive
- We firstly described about the integrated of the newly forming array with a single-shot injection. From this strategy, each extrachromosomal lines are independent and hold different transmission frequency. But, the integration using the existing array is different due to the prior selection of transmitted array before integration process. From this strategy, all generations (P0, F1, F2, and F3) which carry the transmitted array would give (1) the co-injection marker expression with tdTomato background, the consistent proportion of transmitted array in the F2 plates. From the latter, this screening step might required more petri dish for screening.
The integration using integrated Cas9
- The integrated Cas9 is successfully used to encode the Cas9 inside the gonad (Schwartz, PLOS Genetics, 2021). With this transgenic background (oxSi1091[mex-5p::Cas9(smu-2 introns) unc-119(+)] II), the integration of transgene on chromosome II is limited due to the gene proximity. Also, the integrated line from this strategy need to be outcrossed for eliminating Cas9.