Previously, we have talked about the consequences of force on single molecules and on cell. We highlighted some important aspect, how mechanical stress influences development in zebrafish and the worm, and we also mentioned how force might contribute to the etiology of diseases such as cancer. But there is one component missing, one important step that cells need to accomplish before they can react to a force. They need to encounter the force, transmit it, sense it and transduce it. It is conceivable that the physical mechanism of how force acts on cells and how thy sense them is conserved, similar as to the mechanism of how force acts on protein-protein interaction is conserved. Unfortunately, we do not know how force is felt in any system, we hardly know the molecular components that are needed to convert the mechanical stimulus into a stereotypic cellular behavior. Except in a few rare examples such as the little roundworm C elegans. And we think, what makes more sense to study the effect of forces using a system that specifically evolved to detect force and immediately convert them into a cellular signal that can be recorded in real time. We are speaking about the mechanical activation of neurons and the sense of force.
The big advantage is that the cellular consequence can be immediately seen on the depolarization of the neuron; either by electrophysiology, fluorescence activity imaging or a change in behavior of the whole animal. It therefore provides an immediate access to how cells respond to force. For the moment, we are fascinated by the first step of how cells and animals react to mechanical forces – the transmission of mechanical information from the outside to the respective force sensor, which we know is a mechanosensitive ion channel.
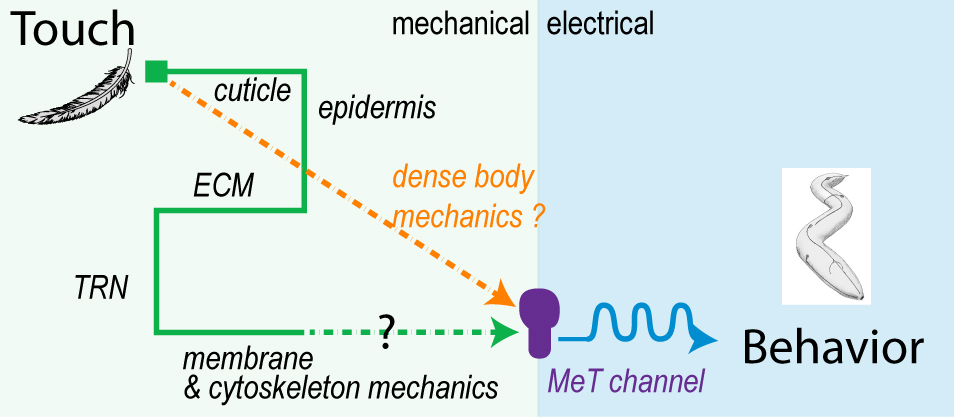
How is touch transferred from the skin to the mechanosensitive ion channel?
To study the force transmission in the whole animal, we use force-sensitive FRET sensor, atomic force microscopy, high resolution Ca2+ microscopy and microfluidics. Our next big goal is to identify the molecular components that are needed to transfer the mechanical energy from the skin to the ion channel. If you want to know more – get excited and stay tuned.
How do mechanosensors remain sensitive despite being continuously mechanically challenged?
Specialized mechanosensitive stretch receptor neurons in our body, called proprioceptors, continuously read out the contractile state of our muscle, position of the joints to the volume of our stomach, blood vessel, lung, bladder and so on. A consequence of their function is, that they get deformed continuously, yet, the remain sensitive to minute mechanical stimuli. Blood vessel expansion, for example is monitored by proprioceptive baroreceptors that are deformed with every heart beat – thus 60 times per minute. Their role is to sense changes in blood pressure and signal to the brain stem to counter act. A remarkably similar situation is found during C elegans locomotion, which propel themselves forward with oscillatory dorso-ventral body waves.
 Thus, not only is the skin, but also its internal organs and neurons subjected to rhythmic mechanical challenges. Moreover, there are specialized mechanosensors, that are proposed to read out these mechanical challenges and signal the state of muscle contraction to the motor-program about the amplitude of the body wave. So far goes the theory, but the details have not been worked out yet.
Thus, not only is the skin, but also its internal organs and neurons subjected to rhythmic mechanical challenges. Moreover, there are specialized mechanosensors, that are proposed to read out these mechanical challenges and signal the state of muscle contraction to the motor-program about the amplitude of the body wave. So far goes the theory, but the details have not been worked out yet.
How do mechanoreceptors remain resilient under continuous mechanical insult?
Good question. Currently, I want to understand what protects neurons from udergoeing extensive buckling and other weird shape instabilities such as hockling. Don’t know what this is?
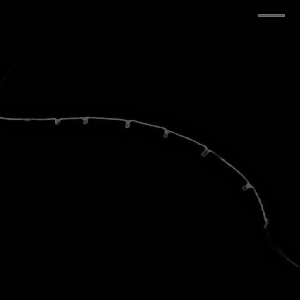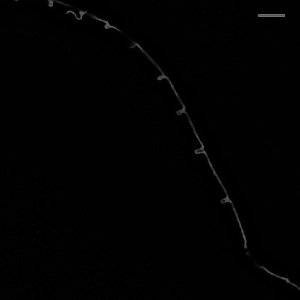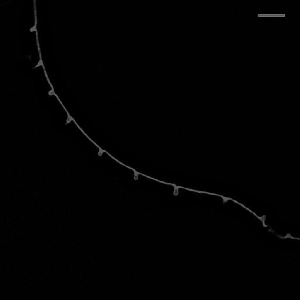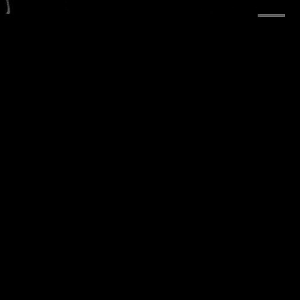 Hockles are 3D deformations of a twisted elastic rod under compression, and those are the structure that develop when you pull your headphone cables out of your pocket. Surprisingly, they also develop in C elegans, in neurons defective in the Alzheimer protein tau and spectrin. We uncovered a role in those two proteins in mechanoprotection and found that they both stabilize the microtubule cytoskeleton and prevent the generation of mechanical torque. We currently believe that tau acts as a lubricant and decorates the individual microtubules and allows for frictionless motion between them when the axon is subjected the mechanical stresses. A failure to do so, leads to a twisted cytoskeleton, torque build-up and in the worst case, axotomies. Perhaps another mechanical origin of neurodegenerative diseases?
Hockles are 3D deformations of a twisted elastic rod under compression, and those are the structure that develop when you pull your headphone cables out of your pocket. Surprisingly, they also develop in C elegans, in neurons defective in the Alzheimer protein tau and spectrin. We uncovered a role in those two proteins in mechanoprotection and found that they both stabilize the microtubule cytoskeleton and prevent the generation of mechanical torque. We currently believe that tau acts as a lubricant and decorates the individual microtubules and allows for frictionless motion between them when the axon is subjected the mechanical stresses. A failure to do so, leads to a twisted cytoskeleton, torque build-up and in the worst case, axotomies. Perhaps another mechanical origin of neurodegenerative diseases?
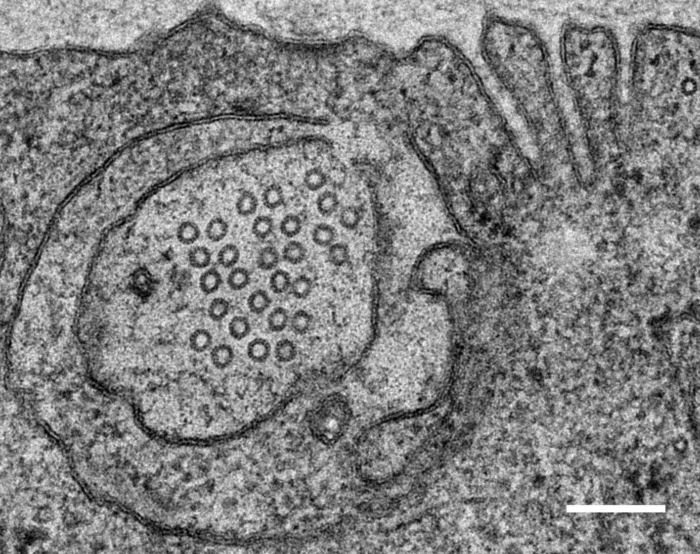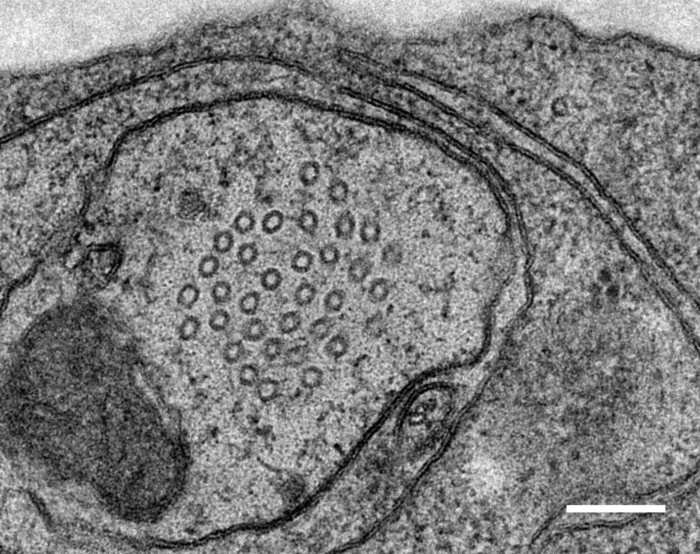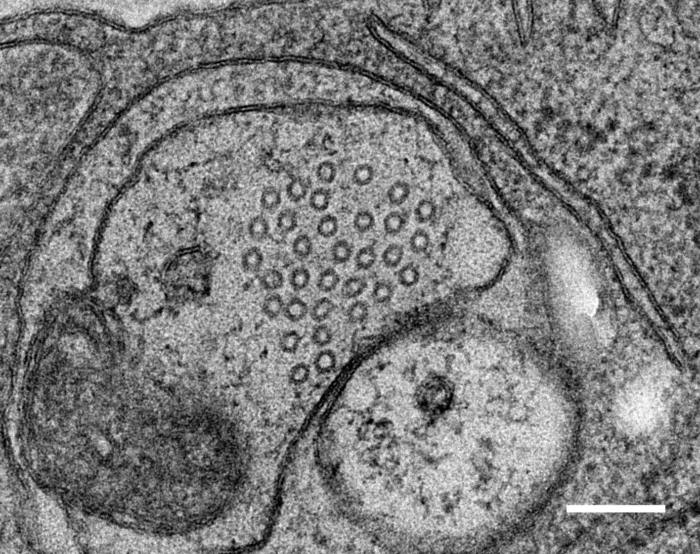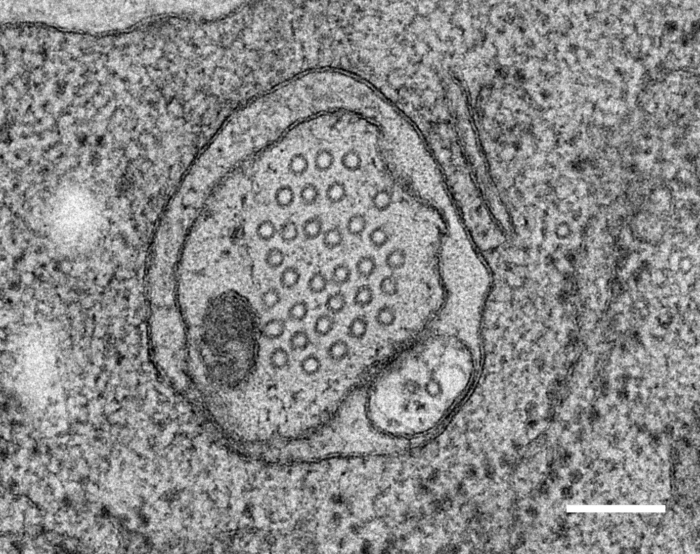
Relevant Publications:
M. Krieg, et al. eLife, 2017
M. Krieg, et al. BioEssays, 2015
M. Krieg, et al. Nat Cell Biol, 16(3), 22433. doi:10.1038/ncb2915, 2014
V. Vazques, et al. Cell Reports, doi:10.1016/j.celrep.2013.12.012, 2013