Light is not only a useful tool for optical imaging and resolving molecular complexes in living cells. It can also be used to infer interactions between two molecular species, mobilities in cellular environments, visualize mechanical tension and activate signaling pathways in neurons. Here you can find a selection of techniques we have used on the past to interrogate our mechanical systems.
Fluoresence recovery after photobleaching (FRAP)
FRAP take advantage of the fact that organic fluorophores can be destroyed by light and thus switch off. The darkened fluorophores get replaced by intact ones over time, resulting in either complete or partial recovery of the fluorescence intensity in this spot. This replacement depends on the diffusion of the fluorophor and how strong it is bound to its environment. Thus, FRAP can be used to measure the mobility of the protein and its binding to a receptor.
Optogenetics and light-controlled behavior using channelrhodopsin ChR2
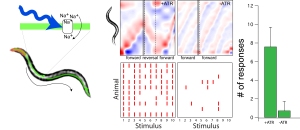
Optogenetic technologies usually refers to methods that use light to control the activity of a neuron or any other cell. Thus, it can be used to steer the behavior of freely moving animals or reconstitute function in neurons that would otherwise be nonfunctional. It is therefore a promising field of research to treat mental disorder in which neuronal excitability is perturbed.
In a first place, our ambitions were not so far-reaching. We used it as a simple test to check whether neurons still function properly and can relay a signal to down stream interneurons in diseased conditions. We expressed the light gated ion channel channelrhodospin in touch receptor neurons that had a defect in mechanosensation and did not respond to a touch applied to its body wall. When stimulated by blue light, animals effectively responded, indicating that light can overcome the mutant mechanosensory defect and that synaptic transmission is functioning normally.
Laser manipulation to visualize neuronal mechanics
Many components in living cells are under isometric tension. Unfortunately, this is hard to see, and one way to visualize this, is to release this tension from the structure under investagation. Similar to what happens if you cut a taut rubber band. When we cut touch receptor neurons with a laser – the two ends retract rapidly. Laser cutting enables tiny, diffraction limited spots to be manipulated and distroyed, which led to the paradigm that actin stress fibers are under constitutive, myosin dependent tension. It is thus a powerful method to manipulated intracellular organelles and infer about their mechanical state.